- Tropospheric ozone
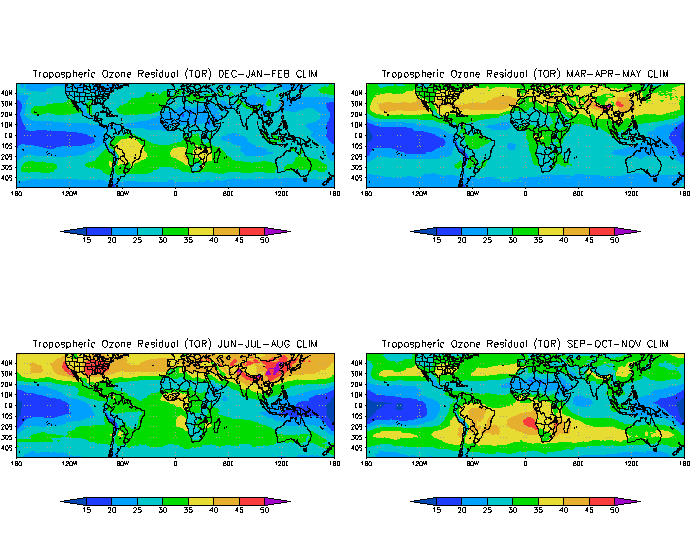
thumb|Seasonal_average_concentrations_of_tropospheric_ozone_in_Dobson unit s over the period 1979 to 2000. In June to August photochemical ozone production causes very high concentrations over the East Coast of the USA and China.]Ozone (O3) is a key constituent of thetroposphere (it is also an important constituent of certain regions of thestratosphere commonly known as theOzone layer ). Photochemical and chemical reactions involving it drive many of the chemical processes that occur in the atmosphere by day and by night. At abnormally high concentrations brought about by human activities (largely the combustion of fossil fuel), it is apollutant , and a constituent ofsmog . Many highly energetic reactions produce it, ranging from combustion to photocopying. Often laser printers will have a smell of ozone, which in high concentrations is toxic. Ozone is a powerfuloxidizing agent readily reacting with other chemical compounds to make many possiblytoxic oxides.The
troposphere extends to between 10 and 18 kilometers above the surface of theEarth and consists of many layers. Ozone is more concentrated above the mixing layer, or ground layer. Ground-level ozone, though less concentrated than ozone aloft, is more of a problem because of its health effects.Tropospheric ozone is a
greenhouse gas and initiates the chemical removal ofmethane and otherhydrocarbon s from the atmosphere. Thus, its concentration affects how long these compounds remain in the air.Satellite s can measure tropospheric ozone. [http://tes.jpl.nasa.gov/] [http://jwocky.gsfc.nasa.gov/] Measurements specifically of ground-level ozone require "in situ" monitoring technology.Formation
The majority of tropospheric ozone formation occurs when
nitrogen oxides (NOx),carbon monoxide (CO) andvolatile organic compounds (VOCs), such asxylene , react in the atmosphere in the presence of sunlight. NOx and VOCs are called ozone precursors. Motor vehicle exhaust, industrial emissions, and chemical solvents are the major anthropogenic sources of these chemicals. Another source is windshield washer fluid. Although these precursors often originate in urban areas, winds can carry NOx hundreds of kilometers, causing ozone formation to occur in less populated regions as well. Methane, a VOC whose atmospheric concentration has increased tremendously during the last century, contributes to ozone formation but on a global scale rather than in local or regional photochemical smog episodes. In situations where this exclusion of methane from the VOC group of substances is not obvious, the term Non-Methane VOC (NMVOC) is often used.The chemical reactions involved in tropospheric ozone formation are a series of complex cycles in which carbon monoxide and VOCs are oxidised to water vapour and carbon dioxide. The reactions involved in this process are illustrated here with CO but similar reactions occur for VOC as well. Oxidation begins with the reaction of CO with the
hydroxyl radical . The hydrogen atom formed by this reacts rapidly with oxygen to give a peroxy radical HO2::OH + CO → H + CO2::H + O2 → HO2
Peroxy radicals then go on to react with NO to give NO2 which is photolysed to give atomic oxygen and through reaction with oxygen a molecule of ozone:
::HO2 + NO → OH + NO2::NO2 + hν → NO + O::O + O2 → O3
The net effect of these reactions is:
::CO + 2O2 → CO2 + O3
This cycle involving HOx and NOx is terminated by the reaction of OH with NO2 to form
nitric acid or by the reaction of peroxy radicals with each other to formperoxides . The chemistry involving VOCs is much more complex but the same reaction of peroxy radicals oxidizing NO to NO2 is the critical step leading to ozone formation.Health effects
Ozone is known to have the following health effects at concentrations common in urban air:
* Irritation of therespiratory system , causing coughing, throat irritation, and/or an uncomfortable sensation in the chest.
* Reducedlung function, making it more difficult to breathe deeply and vigorously. Breathing may become more rapid and more shallow than normal, and a person's ability to engage in vigorous activities may be limited.
* Aggravation ofasthma . When ozone levels are high, more people with asthma have attacks that require a doctor's attention or use of medication. One reason this happens is that ozone makes people more sensitive toallergen s, which in turn trigger asthma attacks.
* Increased susceptibility to respiratory infections.
* Inflammation and damage the lining of the lungs. Within a few days, the damaged cells are shed and replaced much like the skin peels after a sunburn. Animal studies suggest that if this type of inflammation happens repeatedly over a long time period (months, years, a lifetime), lung tissue may become permanently scarred, resulting in permanent loss of lung function and a lower quality of life.A statistical study of 95 large urban communities in the United States found significant association between ozone levels and premature death. The study estimated that a one-third reduction in urban ozone concentrations would save roughly 4000 lives per year (Bell et. al, 2004).
Problem areas
The
United States Environmental Protection Agency has developed an Air Quality index to help explain air pollution levels to the general public. 8-hour average ozone concentrations of 85 to 104 ppbv are described as "Unhealthy for Sensitive Groups", 105 ppbv to 124 ppbv as "unhealthy" and 125 ppb to 404 ppb as "very unhealthy" [http://www.airnow.gov/index.cfm?action=health2.smog1#4] . The EPA has designated over 300 counties of the United States, clustered around the most heavily populated areas (especially in California and the Northeast), as failing to comply with theNational Ambient Air Quality Standards .See also
* Canada-Wide Standards for particulate matter (PM2.5) and ozone [http://www.ccme.ca/assets/pdf/pmozone_standard_e.pdf] (pdf)
*National Ambient Air Quality Standards (USA)
*Ozone
*Photochemical smog
*Troposphere
*Criteria air contaminants References
Michelle L. Bell; Aidan McDermott; Scott L. Zeger; Jonathan M. Samet; Francesca Dominici (2004). "Ozone and Short-term Mortality in 95 US Urban Communities, 1987-2000". Journal of the American Medical Association. 292, 2372-2378.
Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 0-471-17816-0
Wayne, Richard P (2000). Chemistry of Atmospheres (3rd Ed.). Oxford University Press. ISBN 0-19-850375-X
External links
* [http://www.eea.europa.eu/maps/ozone/welcome The European Environment Agency's near real-time ozone map (ozoneweb)]
* [http://www.epa.gov/air/ozonepollution/ U.S. Environmental Protection Agency Ozone Information]
* [http://www.epa.gov/airnow/ U.S. Environmental Protection Agency Live Ozone Map]
* [http://www.epa.gov/oar/oaqps/glo/designations/ U.S. Environmental Protection Agency Ozone Regulation Information]
* [http://www.ucar.edu/learn/1_7_1.htm University Corporation for Atmospheric Research on ozone pollution]
* [http://jwocky.gsfc.nasa.gov/ Total Ozone Mapping Spectrometer] (satellite monitoring)
*WHO-Europe reports: [http://www.who.dk/document/e79097.pdf Health Aspects of Air Pollution (2003)] (PDF) and " [http://www.euro.who.int/document/E82790.pdf Answer to follow-up questions from CAFE (2004)] (PDF)
* [http://www.nasa.gov/vision/earth/environment/ozone_resource_page.html NASA's Ozone Resource Page]
Wikimedia Foundation. 2010.