- Reaction mechanism
-
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.[1]
Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in a reaction mechanism. Recently, electrospray ionization mass spectrometry[2] has been used to corroborate the mechanism of several organic reaction proposals.
Contents
Description
A chemical mechanism describes in detail exactly what takes place at each stage of an overall chemical reaction (transformation). It also describes each reaction intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also account for all reactants used, the function of a catalyst, stereochemistry, all products formed and the amount of each, and what the relative rates of the steps are. Reaction intermediates are chemical species, often unstable and short-lived, which are not reactants or products of the overall chemical reaction, but are temporary products and reactants in the mechanism's reaction steps. Reaction intermediates are often free radicals or ions. Transition states can be unstable intermediate molecular states even in the elementary reactions. Transition states are commonly molecular entities involving an unstable number of bonds and/or unstable geometry which may be at chemical potential maxima.
 SN2 reaction mechanism. Note the negatively-charged transition state in brackets in which the central carbon atom in question shows five bonds, an unstable condition.
SN2 reaction mechanism. Note the negatively-charged transition state in brackets in which the central carbon atom in question shows five bonds, an unstable condition.
The electron or arrow pushing method is often used in illustrating a reaction mechanism; for example, see the illustration of the mechanism for benzoin condensation in the following examples section.
A reaction mechanism must also account for the order in which molecules react. Often what appears to be a single step conversion is in fact a multistep reaction.
Examples
Consider the following reaction:
- CO + NO2 → CO2 + NO
In this case, it has been experimentally determined that this reaction takes place according to the rate law r = k[NO2]2. Therefore, a possible mechanism by which this reaction takes place is:
- 2 NO2 → NO3 + NO (slow)
- NO3 + CO → NO2 + CO2 (fast)
Each step is called an elementary step, and each has its own rate law and molecularity. The elementary steps should add up to the original reaction.
When determining the overall rate law for a reaction, the slowest step is the step that determines the reaction rate. Because the first step (in the above reaction) is the slowest step, it is the rate-determining step. Because it involves the collision of two NO2 molecules, it is a bimolecular reaction with a rate law of r = k[NO2]2. If we were to cancel out all the molecules that appear on both sides of the reaction, we would be left with the original reaction.
In organic chemistry, one of the first reaction mechanisms proposed was that for the benzoin condensation, put forward in 1903 by A. J. Lapworth.
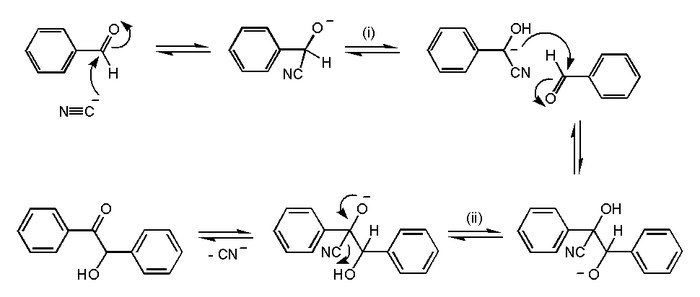 Benzoin condensation reaction mechanism. Cyanide ion (CN-) acts as a catalyst here, entering at the first step and leaving in the last step. Proton (H+) transfers occur at (i) and (ii). The arrow pushing method is used in some of the steps to show where electron pairs go.
Benzoin condensation reaction mechanism. Cyanide ion (CN-) acts as a catalyst here, entering at the first step and leaving in the last step. Proton (H+) transfers occur at (i) and (ii). The arrow pushing method is used in some of the steps to show where electron pairs go.
Modelling
A correct reaction mechanism is an important part of accurate predictive modelling. For many combustion and plasma systems, detailed mechanisms are not available or require development.
Even when information is available, identifying and assembling the relevant data from a variety of sources, reconciling discrepant values and extrapolating to different conditions can be a difficult process without expert help. Rate constants or thermochemical data are often not available in the literature, so computational chemistry techniques or group-additivity methods must be used to obtain the required parameters.
At the different stages of a reaction mechanism's elaboration, appropriate methods must be used.
Molecularity
Main article: molecularityMolecularity in chemistry is the number of colliding molecular entities that are involved in a single reaction step.
- A reaction involving one molecular entity is called unimolecular.
- A reaction involving two molecular entities is called bimolecular.
- A reaction involving three molecular entities is called termolecular.
See also
- Organic reactions by mechanism under Organic reaction
- Nucleophilic acyl substitution
- Neighbouring group participation
- Finkelstein reaction
- Lindemann mechanism
- Electrochemical reaction mechanism
- Arrow pushing
References
- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ^ Investigation of chemical reactions in solution using API-MS Leonardo Silva Santos, Larissa Knaack, Jurgen O. Metzger Int. J. Mass Spectrom.; 2005; 246 pp 84 - 104; (Review) doi:10.1016/j.ijms.2005.08.016
Basic reaction mechanisms Nucleophilic substitution Elimination reaction Unimolecular elimination (E1) · E1cB elimination reaction · Bimolecular elimination (E2)Related topics Chemical kinetics Categories:- Chemical reactions
- Reaction mechanisms
- Chemical kinetics
- Chemical engineering
- Combustion
Wikimedia Foundation. 2010.
