- Metal carbonyl
-
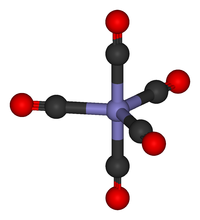
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl (Ni(CO)4), but more commonly metal carbonyls contain a mix of ligands, such as Re(CO)3(2,2'-bipyridine)Cl. Carbon monoxide is an important building block for the synthesis of many compounds, for example hydroformylation, and metal carbonyl catalysts are central to its utilization. Metal carbonyls are toxic, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which will not bind O2.[1]
Contents
Structure and properties
Metal carbonyls generally have poor solubility with water.
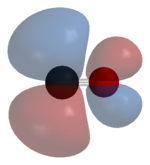
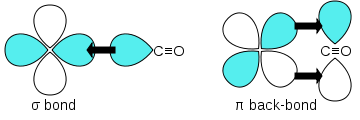
Carbon monoxide bonds to transition metals using "synergic π* back-bonding." The bonding has three components, giving rise to a partial triple bond. A sigma bond arises from overlap of nonbonding electron pair on carbon with a blend of d-, s-, and p-orbitals on the metal. A pair of π bonds arises from overlap of filled d-orbitals on the metal with a pair of π-antibonding orbitals projecting from the carbon of the CO. The latter kind of binding requires that the metal have d-electrons, and that the metal is in a relatively low oxidation state (<+2). The π-bonding has the effect of weakening the carbon-oxygen bond compared with free carbon monoxide. Because of the multiple bond character of the M-CO linkage, the distance between the metal and carbon is relatively short, often < 1.8 Â, about 0.2 Â shorter than a metal-alkyl bond.
Bonding modes in clusters
The carbonyl ligand engages in a range of bonding modes in metal carbonyl cluster chemistry.[1][2] Most frequently CO binds in the familiar terminal mode (see above), but CO often bridges between two (μ2) or three (μ3) metals. Much less common are bonding modes in which both C and O bond to the metal, e.g. μ3-η2.
The increased π-bonding due to back-donation from multiple metal centers results in further weakening of the C-O bond.
Characterization
Compound νCO (cm-1) CO 2143 Ti(CO)6-2 1748 V(CO)6-1 1859 Cr(CO)6 2000 Mn(CO)6+ 2100 Fe(CO)62+ 2204 Fe(CO)5 2022, 2000 The most important technique for characterizing metal carbonyls is infra-red spectroscopy. The C-O vibration, typically called νCO, occurs at 2143 cm-1 for CO gas. The positions of the νCO band(s) for the metal carbonyls is inversely correlated with the strength of the pi-bonding between the metal and the carbon:
In addition to their frequency, the number of the νCO bands is diagnostic of structure of the complex. Octahedral complexes, e.g. Cr(CO)6, exhibits only a single νCO band in its IR spectrum. Spectra for complexes of lower symmetry are more complex. For example, the IR spectrum of Fe2(CO)9 displays CO bands at 2082, 2019, 1829 cm-1.
In cluster carbonyls, νCO is a sensitive probe for the CO coordination geometry. For bridging (μ2) ligands νCO is usually shifted by 100-200 cm-1 to lower wavenumbers compared to the signatures of μ1-CO. Bands for face capping (μ3) CO ligands appear at even lower energies. Typical values for rhodium cluster carbonyls are:[3]
carbonyl νCO, µ1 (cm-1) νCO, µ2 (cm-1) νCO, µ3 (cm-1) Rh2(CO)8 2060, 2084 1846, 1862 Rh4(CO)12 2044, 2070, 2074 1886 Rh6(CO)16 2045, 2075 1819 Synthesis
Although nickel carbonyl and iron pentacarbonyl form upon treatment of the metals with carbon monoxide, most metal carbonyls are prepared less directly. The other homoleptic carbonyls are usually made by "reductive carbonylation" of metal salts or metal oxides under a high pressure of carbon monoxide in autoclave:
- Re2O7 + 17 CO → Re2(CO)10 + 7 CO2
Once prepared, these homoleptic carbonyls undergo extensive substitution and redox reactions.
Mixed ligand carbonyls of ruthenium, osmium, rhodium, and iridium are often generated by abstraction of CO from solvents such as dimethylformamide (DMF) and 2-methoxyethanol. Typical is the synthesis of IrCl(CO)(PPh3)2 from the reaction of iridium(III) chloride and triphenylphosphine in boiling DMF solution.
Occurrence in nature
The hydrogenase enzymes contain CO bound to iron, apparently the CO stabilizes low oxidation states which facilitates the binding of hydrogen.[4] Certain metal carbonyls have been observed in trace amounts in landfills, where the reducing environment is compatible with their formation.[5]
Compounds
Most metal carbonyl complexes contain a mixture of ligands. Examples include the historically important IrCl(CO)(P(C6H5)3)2 and the anti-knock agent (CH3C5H4)Mn(CO)3. The parent compounds for many of these mixed ligand complexes are the binary carbonyls, i.e. species of the formula [M(CO)n]z, many of which are commercially available. The formula of many metal carbonyls can be inferred from the 18 electron rule.
Charge-neutral binary metal carbonyls
- Group 4 elements with 4 valence electrons are rare, but substituted derivatives of Ti(CO)7 are known.
- Group 5 elements with 5 valence electrons, again are subject to steric effects that prevent the formation of M-M bonded species such as V2(CO)12, which is unknown. The 17 VE V(CO)6 is however well known.
- Group 6 elements with 6 valence electrons form metal carbonyls Cr(CO)6, Mo(CO)6, and W(CO)6 (6 + 6x2 = 18 electrons).
- Group 7 elements with 7 valence electrons form metal carbonyl dimers Mn2(CO)10, Tc2(CO)10, and Re2(CO)10 (7 + 1 + 5x2 = 18 electrons).
- Group 8 elements with 8 valence electrons form metal carbonyls Fe(CO)5,Ru(CO)5 and Os(CO)5 (8 + 5x2 = 18 electrons). The heavier two members are unstable, tending to decarbonylate to give Ru3(CO)12, and Os3(CO)12. The two other principal iron carbonyls are Fe3(CO)12 and Fe2(CO)9.
- Group 9 elements with 9 valence electrons and are expected to form metal carbonyl dimers M2(CO)8. In fact the cobalt derivative of this octacarbonyl is the only stable member, but all three tetramers are well known: Co4(CO)12, Rh4(CO)12, Rh6(CO)16, and Ir4(CO)12 (9 + 3 + 3x2 = 18 electrons). Co2(CO)8 unlike the majority of the other 18 VE transition metal carbonyls is sensitive to oxygen.
- Group 10 elements with 10 valence electrons form metal carbonyls Ni(CO)4 (10 + 4x2 = 18 electrons). Curiously Pd(CO)4 and Pt(CO)4 are not stable.
Anionic binary metal carbonyls
- Group 4 elements as dianions resemble neutral group 6 derivatives: [Ti(CO)6]2-.[6]
- Group 5 elements as monoanions resemble again neutral group 6 derivatives: [V(CO)6]-.
- Group 7 elements as monoanions resemble neutral group 8 derivatives: [M(CO)5]- (M = Mn, Tc, Re).
- Group 8 elements as dianaions resemble neutral group 10 derivatives: [M(CO)4]2- (M = Fe, Ru, Os). Condensed derivatives are also known.
- Group 9 elements as monoanions resemble neutral group 10 metal carbonyl. [Co(CO)4]- is the best studied member.
Large anionic clusters of Ni, Pd, and Pt are also well known.
Cationic binary metal carbonyls
- Group 7 elements as monocations resemble neutral group 6 derivative [M(CO)6]+ (M = Mn, Tc, Re).
- Group 8 elements as dications also resemble neutral group 6 derivatives [M(CO)6]2+ (M = Fe, Ru, Os).[7]
Metal carbonyl hydrides
Metal Carbonyl hydride pKa HCo(CO)4 "strong" HCo(CO)3(P(OPh)3) 5.0 HCo(CO)3(PPh3) 7.0 HMn(CO)5 7.1 H2Fe(CO)4 4.4, 14 [HCo(dmgH)2PBu3 10.5 Metal carbonyls are relatively distinctive in forming complexes with negative oxidation states. Examples include the anions discussed above. These anions can be protonated to give the corresponding metal carbonyl hydrides. The neutral metal carbonyl hydrides are often volatile and can be quite acidic.[8]
Related compounds
Many ligands are known to form homoleptic and mixed ligand complexes that are analogous to the metal carbonyls.
Complexes of nitrosyls
Metal nitrosyls, featuring NO as a ligand are numerous, although homoleptic derivatives are not. Relative to CO, NO is a stronger acceptor and isocyanides are better donors. Well known nitrosyl carbonyls include CoNO(CO)3 and Fe(NO)2(CO)2.[9]
Complexes of thiocarbonyls
Complexes containing CS are known but are uncommon.[10][11] The rarity of such complexes is attributable in part to the fact that the obvious source material, carbon monosulfide, is unstable. Thus, the synthesis of thiocarbonyl complexes requires more elaborate routes, such as the reaction of disodium tetracarbonylferrate with thiophosgene:
- Na2Fe(CO)4 + CSCl2 → Fe(CO)4CS + 2 NaCl
Complexes of CSe and CTe are very rare.
Complexes of phosphines
All metal carbonyls undergo substitution by organophosphorus ligands. For example, the series Fe(CO)5-x(PR3)x is well known for various phosphine ligands for x = 1, 2, and 3. PF3 behaves similarly but is remarkable because it readily forms homoleptic analogues of the binary metal carbonyls. For example the volatile, stable complexes Fe(PF3)5 and Co2(PF3)8 represent CO-free analogues of Fe(CO)5 and Co2(CO)8 (unbridged isomer).
Complexes of isocyanides
Isocyanides also form extensive families of complexes that are related to the metal carbonyls. Typical isocyanide ligands are MeNC and t-butylisocyanide ((Me3CNC). A special case is CF3NC, an unstable molecule that forms stable complexes whose behavior closely parallels that of the metal carbonyls.
History
Initial experiments on the reaction of carbon monoxide with metals were carried out by Justus von Liebig in 1834. By passing carbon monoxide over molten potassium he prepared a substance having the empirical formula KCO, which he called Kohlenoxidkalium.[12] As demonstrated later, the compound was not a metal carbonyl, but the potassium salt of ´hexahydroxy benzene and the potassium salt of dihydroxy acetylene[13]
The synthesis of the first true, however, heteroleptic metal carbonyl complex was performed by Paul Schützenberger in 1868 by passing chlorine and carbon monoxide over platinum black, where Dicarbonyl dichloro platinum (Pt(CO)2Cl2) was formed.[14]
Ludwig Mond, one of the founders of Imperial Chemical Industries, investigated in the 1890s with Carl Langer and Friedrich Quincke various processes for the recovery of chlorine which was lost in the Solvay process by nickel metals, oxides and salts. [13] As part of their experiments the group treated nickel with carbon monoxide. They found that the resulting gas colored the gas flame of a burner in a greenish-yellowish color; when heated in a glass tube it formed a nickel mirror. The gas could be condensed to a colorless, water-clear liquid with a boiling point of 43 °C. Thus, Mond and his coworker had discovered the first pure, homoleptic metal carbonyl, nickel tetracarbonyl (Ni(CO)4).[15] The unusual high volatility of the metal compound nickel tetracarbonyl led Kelvin with the statement that Mond had "given wings to the metals' [16].
The following year, Mond and Marcellin Berthelot independently discovered iron pentacarbonyl, which is produced by a similar procedure as nickel tetracarbonyl. Mond recognized the economic potential of this class of compounds, which he commercially used in the Mond process and financed more research on related compounds. Heinrich Hirtz and his colleague M. Dalton Cowap synthesized metal carbonyls of cobalt, molybdenum, ruthenium, and diiron nonacarbonyl.[17]
The economic benefits of metal-catalysed carbonylations, e.g. Reppe chemistry and hydroformylation, led to growth of the area. Metal carbonyl compounds were discovered in the active sites of three naturally occurring enzymes.[18]
See also
References
- ^ a b Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-29390-6
- ^ P. J. Dyson and J. S. McIndoe, Transition Metal Carbonyl Cluster Chemistry, Gordon & Breach: Amsterdam (2000). ISBN 9056992899.
- ^ A.D. Allian, Y. Wang, M. Saeys, G.M. Kuramshina, M. Garland (2006). "The combination of deconvolution and density functional theory for the mid-infrared vibrational spectra of stable and unstable rhodium carbonyl clusters". Vibrational Spectroscopy 41: 101–111. doi:10.1016/j.vibspec.2006.01.013.
- ^ Bioorganometallics: Biomolecules, Labeling, Medicine; Jaouen, G., Ed. Wiley-VCH: Weinheim, 2006.3-527-30990-X.
- ^ Feldmann, J. (1999). "Determination of Ni(CO)4, Fe(CO)5, Mo(CO)6, and W(CO)6 in sewage gas by using cryotrapping gas chromatography inductively coupled plasma mass spectrometry". Journal of Environmental Monitoring 1: 33–37. doi:10.1039/a807277i.
- ^ Ellis, J. E. (2003). "Metal Carbonyl Anions: from [Fe(CO)4]2- to [Hf(CO)6]2- and Beyond". Organometallics 22 (17): 3322–3338. doi:10.1021/om030105l.
- ^ Finze, M.; Bernhardt, E.; Willner, H.; Lehmann, C. W.; Aubke, F. (2005). "Homoleptic, σ-Bonded Octahedral Superelectrophilic Metal Carbonyl Cations of Iron(II), Ruthenium(II), and Osmium(II). Part 2: Syntheses and Characterizations of [M(CO)6][BF4]2 (M = Fe, Ru, Os)". Inorg. Chem. 44 (12): 4206–4214. doi:10.1021/ic0482483. PMID 15934749.
- ^ Pearson, Ralph G. (1995). "The Transition-Metal-Hydrogen Bond". Chem. Rev. 95: 41. doi:10.1021/cr00065a002.
- ^ Hayton, T. W.; Legzdins, P. and Sharp, W. B., "Coordination and Organometallic Chemistry of Metal-NO Complexes", Chemical Reviews, 2002, volume 102, 935-991.doi:10.1021/cr000074t
- ^ Petz, W., "40 years of transition-metal thiocarbonyl chemistry and the related CSe and CTe compounds", Coordination Chemistry Reviews, 2008, 252, 1689-1733.doi:10.1016/j.ccr.2007.12.011.
- ^ Hill, A. F.; Wilton-Ely, J. D. E. T. (2002). "Chlorothiocarbonyl-bis(triphenylphosphine) iridium(I) [IrCl(CS)(PPh3)2]". Inorg. Synth. 33: 244–245. doi:10.1002/0471224502.ch4. ISBN 0471208256.
- ^ WE Trout.The metal carbonyls, in: J. Chem Educ .,1937,14 (10), p. 453; doi:10.1021/ed014p453.
- ^ a b W. A. Herrmann:100 Jahre Metallcarbonyle, in:Chemie in unserer Zeit,1988, 22 Jahrg, No. 4, p. 113-122, ISSN 0009-2851 .
- ^ P. Schützenberger,Ann. Chim. .Phys.(Paris),1868,15, p. 100-106.
- ^ L. Mond, C. Langer, F. Quincke: Action of carbonyls Monoxide on Nickel, in:J. Chem Soc .,1890,57, p. 749-753; doi:10.1039/CT8905700749.
- ^ W. Gratzer: Metal Takes Wing in: Eureka and Euphorias: The Oxford Book of Scientific Anecdotes, 320 pages, published by Oxford University Press (2002) ISBN 0-19280403-0
- ^ L. Mond, H. Hirtz, MD Cowap: Note on a volatile compound of cobalt with Carbon Monoxide, Chem News, vol. 98, 1908, p. 165; Chem Abs, vol. 2,1908, p. 3315
- ^ C. Tard, C. J. Pickett, Structural and Functional Analogues of the Active Sites of the [Fe]-, [NiFe]-, and [FeFe]-Hydrogenases Chem. Rev. 2009, 109, 2245-2274. doi:10.1021/cr800542q
External links
Categories:- Carbonyl complexes
- Organometallic chemistry
- Transition metals
Wikimedia Foundation. 2010.