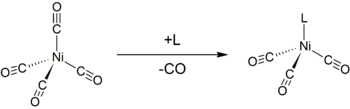- Nickel tetracarbonyl
-
Nickel tetracarbonyl 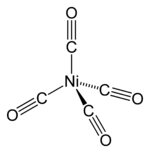
 TetracarbonylnickelOther namesNickel carbonyl
TetracarbonylnickelOther namesNickel carbonylIdentifiers CAS number 13463-39-3 
PubChem 26039 ChemSpider 21865021 
EC number 236-669-2 UN number 1259 ChEBI CHEBI:30372 
RTECS number QR6300000 Jmol-3D images Image 1 - C(#O)[Ni](C#O)(C#O)C#O
Properties Molecular formula Ni(CO)4 Molar mass 170.73 g/mol Appearance colorless or very-pale-yellow liquid
diamagneticDensity 1.319 g/cm3 Melting point -17.2 °C, 256 K, 1 °F
Boiling point 43 °C, 316 K, 109 °F
Solubility in water .018 g/100 mL (10 °C) Solubility miscible in most organic solvents
soluble in nitric acid, aqua regiaViscosity 3.05 x 10-4 Pa s Structure Coordination
geometryTetrahedral Molecular shape Tetrahedral Dipole moment zero Thermochemistry Std enthalpy of
formation ΔfHo298−632 kJ/mol Std enthalpy of
combustion ΔcHo298−1180 kJ/mol Standard molar
entropy So298320 J K−1 mol−1 Hazards MSDS ICSC 0064 EU Index 028-001-00-1 EU classification Flammable ('F)
Carc. Cat. 3
Repr. Cat. 2
Very Toxic (T+)
Dangerous for the environment (N)R-phrases R61, R11, R26, R40, R50/53 S-phrases S53, S45, S60, S61 NFPA 704 Flash point 4 °C Autoignition
temperature60 °C Explosive limits 2–34% Related compounds Related metal carbonyls Iron pentacarbonyl
Dicobalt octacarbonyl tetracarbonyl (verify) (what is:
tetracarbonyl (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nickel carbonyl (IUPAC name: tetracarbonylnickel) is the organonickel compound with the formula Ni(CO)4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry. Nickel carbonyl is the most toxic substance encountered in industrial processes.[1]
Contents
Structure and bonding
Nickel tetracarbonyl contains nickel in the zero oxidation state. It has 18 valence electron, and is a tetrahedral molecule with four carbonyl (carbon monoxide) ligands attached to nickel. The CO ligands, in which the C and the O are connected by triple bonds (often depicted as double bonds), are covalently bonded to the nickel atom via the carbon ends. Electron diffraction studies have been performed on this molecule, and the Ni-C and C-O distances have been calculated to be 1.838(2) and 1.141(2) angstroms, respectively.[2]
Preparation
Ni(CO)4 was first synthesised in 1890 by Ludwig Mond by the direct reaction of nickel metal with CO.[3] This pioneering work foreshadowed the existence of many other metal carbonyl compounds, including those of V, Cr, Mn, Fe, and Co. It was also applied industrially to the purification of nickel by the end of the 19th century.[4]
At 323 K (50 °C (122 °F)), carbon monoxide is passed over impure nickel. The optimal rate occurs at 130 °C.[5]
Ni(CO)4 is not readily available commercially. It is conveniently generated in the laboratory by carbonylation of commercially available bis(cyclooctadiene)nickel(0).
Reactions
Thermal decarbonylation
On moderate heating, Ni(CO)4 decomposes to carbon monoxide and nickel metal. Combined with the easy formation from CO and even impure nickel, this decomposition is the basis for the Mond process for the purification of nickel. Thermal decomposition commences near 180 °C and increases at higher temperature[5]
Reactions with nucleophiles and reducing agents
Like other low-valent metal carbonyls, Ni(CO)4 is susceptible to attack by nucleophiles. Attack can occur at nickel center, resulting in displacement of CO ligands, or at CO. Thus, donor ligands such as triphenylphosphine react to give Ni(CO)3(PPh3) and Ni(CO)2(PPh3)2. Bipyridine and related ligands behave similarly.[6] The monosubstitution of nickel tetracarbonyl with other ligands can be used to determine the Tolman electronic parameter, a measure of the electron donating or withdrawing ability of a given ligand.
Treatment with hydroxides gives clusters such as [Ni5(CO)12]2− and [Ni6(CO)12]2−. These compounds can also be obtained by reduction of nickel carbonyl.
Thus, treatment of Ni(CO)4 with carbon nucleophiles (Nu−) results in acyl derivatives such as [Ni(CO)3C(O)Nu)]−.[7]
Reactions with electrophiles and oxidizing agents
Nickel carbonyl can be oxidized. Chlorine oxidizes nickel carbonyl into NiCl2, releasing CO gas. Other halogens behave analogously. This reaction provides a convenient method for destroying unwanted portions of the toxic compound.
Reactions of Ni(CO)4 with alkyl and aryl halides often result in carbonylated organic products. Vinylic halides, such as PhCH=CHBr, are converted to the unsaturated esters upon treatment with Ni(CO)4 followed by sodium methoxide. Such reactions also probably proceed via oxidative addition. Allylic halides give the pi-allyl nickel compounds, such as (allyl)2Ni2Cl2:[8]
- 2 Ni(CO)4 + 2 ClCH2CH=CH2 → Ni2(μ-Cl)2(η3-C3H5)2 + 8 CO
Toxicology and safety considerations
Ni(CO)4 is highly hazardous, much more so than implied by its CO content, reflecting the effects of the nickel if it were released in the body. Nickel carbonyl may be fatal if absorbed through the skin or more likely, inhaled due to its high volatility. Its LC50 for a 30-minute exposure has been estimated at 3 ppm, and the concentration that is immediately fatal to humans would be 30 ppm. Some subjects exposed to puffs up to 5 ppm described the odour as musty or sooty, but since the compound is so exceedingly toxic its smell provides no reliable warning against a potentially fatal exposure.[9] Historically, laboratories that used Ni(CO)4 would keep a canary in the lab as an indicator of nickel carbonyl toxicity, due to the higher sensitivity of birds to this poison.[citation needed]
Human systemic effects by inhalation: somnolence, fever, and other pulmonary changes. Vapors may cause coughing, dyspnea (difficult breathing), irritation, congestion and edema of the lungs, tachycardia (rapid pulse), cyanosis, headache, dizziness, and weakness. Toxicity by inhalation is believed to be caused by both the nickel and carbon monoxide liberated in the lungs. Chronic exposure may cause cancer of lungs or nasal sinuses. Sensitization dermatitis is fairly common. It is considered the most hazardous compound of nickel in the workplace. It is lipid soluble and can cross biological membranes (e.g., lung alveolus, blood-brain barrier, placental barrier).
The vapours of Ni(CO)4 can autoignite.
Nickel carbonyl poisoning is characterized by a two-stage illness. The first consists of headaches and chest pain lasting a few hours, usually followed by a short remission. The second phase is a chemical pneumonitis which starts after typically 16 hours with symptoms of cough, breathlessness and extreme fatigue. These reach greatest severity after four days, possibly resulting in death from cardiorespiratory or renal failure. Convalescence is often extremely protracted, often complicated by exhaustion, depression and dyspnea on exertion. Permanent respiratory damage is unusual. The carcinogenicity of Ni(CO)4 is a matter of debate.
Nickel carbonyl vapor decomposes quickly in air, lasting only about a minute.[10]
References
- ^ The Merck Index. Merck. Seventh Edition.
- ^ Hedberg, Lise; Iijima, Takao; Hedberg, Kenneth (1979). "Nickel tetracarbonyl, Ni(CO)4. I. Molecular structure by gaseous electron diffraction. II. Refinement of quadratic force field". The Journal of Chemical Physics 70 (7): 3224. doi:10.1063/1.437911.
- ^ Mond, L.; Langer, C.; Quincke, F. (1890). "Action of Carbon Monoxide on Nickel". J. Chem. Soc., Trans. 57: 749–53. doi:10.1039/CT8905700749.
- ^ "The Extraction of Nickel from its Ores by the Mond Process". Nature 59 (1516): 63–64. 1898. doi:10.1038/059063a0.
- ^ a b Lascelles,Keith; Morgan, Lindsay G.; & Nicholls, David (1991). "Nickel Compounds". Ullmann's Encyclopedia of Industrial Chemistry A17 (5): 235–249. doi:10.1002/14356007.a17_157. ISBN 3527306730.
- ^ Elschenbroich, C.; Salzer, A. (1992). Organometallics : A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
- ^ EROS Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, 2003.
- ^ Semmelhack, M. F.; Helquist, P. M. (1988), "Reaction of Aryl Halides with π-Allylnickel Halides: Methallylbenzene", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv6p0722; Coll. Vol. 6: 722
- ^ Board on Environmental Studies and Toxicology (2008). "Nickel Carbonyl: Acute Exposure Guideline Levels". Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6. National Academies Press. pp. 213–259. http://books.nap.edu/openbook.php?record_id=12018&page=159.
- ^ Stedman, D. H.; Hikade, D. A.; Pearson, Jr., R.; Yalvac, E. D. (1980). "Nickel Carbonyl: Decomposition in Air and Related Kinetic Studies". Science 208 (4447): 1029–1031. doi:10.1126/science.208.4447.1029. PMID 17779026.
Further reading
- Shi Z (1991). "Nickel carbonyl: toxicity and human health". The Science of the Total Environment 148 (2–3): 293–298. doi:10.1016/0048-9697(94)90406-5. PMID 8029705.
- Sunderman FW (1989). "A Pilgrimage into the Archive of Nickel Toxicology". Annals of Clinical and Lalboratory Science 19: 1–16.
- Armit HW (1908). "The toxicology of nickel carbonyl. Part II". Journal of Hygiene 8 (5): 565–610. PMC 2167169. PMID 20474374. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2167169.
- Armit HW (1907). "The toxicology of nickel carbonyl". Journal of Hygiene 7 (4): 525–551. doi:10.1017/S0022172400033507. PMC 2236193. PMID 20474327. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2236193.
- Barceloux DG; Barceloux, Donald (1999). "Nickel". Journal of Toxicology-Clinical Toxicology 37 (2): 239–258. doi:10.1081/CLT-100102423. PMID 10382559. DOI
External links
- International Chemical Safety Card 0064
- National Pollutant Inventory - Nickel carbonyl fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph "Nickel and Nickel compounds"
Nickel compounds Categories:- Organonickel compounds
- Carbonyl complexes
- IARC Group 1 carcinogens
Wikimedia Foundation. 2010.