- Dicobalt octacarbonyl
-
Dicobalt octacarbonyl 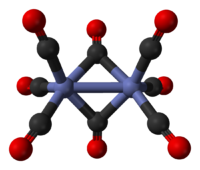
 Octacarbonyldicobalt(Co—Co)Other namesCobalt carbonyl
Octacarbonyldicobalt(Co—Co)Other namesCobalt carbonylIdentifiers CAS number 10210-68-1 
ChemSpider 2007057 
UN number 3281 RTECS number GG0300000 Jmol-3D images Image 1 - [Co+2].[Co+2].[C-]#[O+].[O+]#[C-].[O+]#[C-].[O+]#[C-].[O+]#[C-].[O+]#[C-].[O+]#[C-].[O+]#[C-]
Properties Molecular formula Co2(CO)8 Molar mass 341.95 g/mol Appearance red-orange crystals
when pureDensity 1.87 g/cm3 Melting point 51-52 °C, 324-325 K, 124-126 °F
Boiling point 52 °C, 325 K, 126 °F (decomp. ca.)
Solubility in water insoluble Structure Dipole moment 1.33 D (C2v isomer)
0 D (D3d isomer)Hazards MSDS External MSDS Main hazards Toxic Related compounds Related metal carbonyls Iron pentacarbonyl
Diiron nonacarbonyl
Nickel tetracarbonyl (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dicobalt octacarbonyl is the inorganic compound Co2(CO)8. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis.[1] It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes.[2]
Contents
Synthesis, structure, properties
The high pressure carbonylation of cobalt(II) salts, often in the presence of cyanide, affords this compound. It is an orange-colored, pyrophoric solid that is thermally unstable. It exists as two isomers in solution:[3]
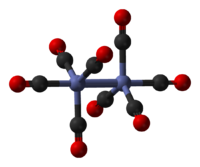
The predominant isomer resembles Fe2(CO)9, less one bridging CO. The Co-Co distance is 2.52 Å, and the Co-COterminal and Co-CObridge distances are 1.80 and 1.90 Å, respectively.[4] These isomers rapidly interconvert. The minor isomer has no bridging CO ligands; it is described (CO)4Co-Co(CO)4 (D3d symmetry group). The major isomer contains two bridging CO ligand and features octahedral cobalt, describable as (CO)3Co(μ-CO)2Co(CO)3 (C2v symmetry group). The minor isomer has been crystallized together with C60.[5]
Reactions
The most characteristic reaction of Co2(CO)8 is its hydrogenation to tetracarbonylhydrocobalt, [HCo(CO)4]:
- Co2(CO)8 + H2 → 2 HCo(CO)4
This hydride is the active agent in hydroformylation. It adds to alkenes to give an alkyl-Co(CO)4 product that then proceeds to insert CO and undergo hydrogenolysis to afford the aldehyde. Reduction of Co2(CO)8 gives the conjugate base of HCo(CO)4:
- Co2(CO)8 + 2 Na → 2 NaCo(CO)4
The CO ligands can be replaced with tertiary phosphine ligands to give Co2(CO)8-x(PR3)x. These bulky derivatives are more selective catalysts for hydroformylation reactions. "Hard" Lewis bases, e.g. pyridine, cause disproportionation:
- 6 C6H5N + 1.5 Co2(CO)8 → [Co(C6H5N)6][Co(CO)4]2 + 4 CO
Co2(CO)8 catalyzes the Pauson–Khand reaction of an alkyne, an alkene, and CO to give cyclopentenones.
Heating causes decarbonylation and formation of the tetrahedral cluster:
- 2 Co2(CO)8 → Co4(CO)12 + 4 CO
Safety
Co2(CO)8 a volatile source of cobalt(0), is pyrophoric and releases carbon monoxide upon decomposition.[6]
See also
References
- ^ Pauson, P. L. “Octacarbonyldicobalt” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289[dead link].
- ^ Elschenbroich, C.; Salzer, A. ”Organometallics : A Concise Introduction” (2nd Ed) (1992) Wiley-VCH: Weinheim. ISBN 3-527-28165-7
- ^ Ray L. Sweany and Theodore L. Brown "Infrared spectra of matrix-isolated dicobalt octacarbonyl. Evidence for the third isomer" Inorganic Chemistry 1977, 16, pp 415 - 421. doi:10.1021/ic50168a037
- ^ G.G. Sumner, HP Klug, LE Alexander "The crystal structure of dicobalt octacarbonyl" Acta Crystallographica, 1964 Volume 17 Part 6 Pages 732-742. doi:10.1107/S0365110X64001803
- ^ "Splendid symmetry: crystallization of an unbridged isomer of Co2(CO)8 in Co2(CO)8·C60" Thelma Y. Garcia, James C. Fettinger, Marilyn M. Olmstead, Alan L. Balch, Chem. Commun., 2009. doi:10.1039/b915083h.
- ^ Cole Parmer MSDS
Categories:- Carbonyl complexes
- Organocobalt compounds
Wikimedia Foundation. 2010.

