- Redox
-
Redox (portmanteau for reduction-oxidation) reactions describe all chemical reactions in which atoms have their oxidation state changed. This can be either a simple redox process, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), or a complex process such as the oxidation of glucose (C6H12O6) in the human body through a series of complex electron transfer processes.
Redox reactions, or oxidation-reduction reactions, have a number of similarities to acid-base reactions. Fundamentally, redox reactions are a family of reactions that are concerned with the transfer of electrons between species. Like acid-base reactions, redox reactions are a matched set, that is there cannot be an oxidation reaction without a reduction reaction happening simultaneously. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. Each reaction by itself is called a "half-reaction", simply because there must be two half-reactions to form a whole reaction. Thus, in notating redox reactions, chemists typically write out the electrons explicitly:
The term comes from the two concepts of reduction and oxidation. It can be explained in simple terms:
- Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion.
- Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
Though sufficient for many purposes, these descriptions are not precisely correct. Oxidation and reduction properly refer to a change in oxidation state — the actual transfer of electrons may never occur. Thus, oxidation is better defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as "redox" even though no electron transfer occurs (such as those involving covalent bonds).
Non-redox reactions, which do not involve changes in formal charge, are known as metathesis reactions.
 Rusting iron
Rusting iron
Contents
Oxidizing and reducing agents
In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or reducing agent loses electrons and is oxidized, and the oxidant or oxidizing agent gains electrons and is reduced. The pair of an oxidizing and reducing agent that are involved in a particular reaction is called a redox pair.
Oxidizers
Substances that have the ability to oxidize other substances are said to be oxidative or oxidizing and are known as oxidizing agents, oxidants, or oxidizers. Put another way, the oxidant (oxidizing agent) removes electrons from another substance; i.e., it oxidizes other substances, and is thus itself reduced. And, because it "accepts" electrons, it is also called an electron acceptor.
Oxidants are usually chemical elements or substances with elements in high oxidation states (e.g., H2O2, MnO−
4, CrO3, Cr2O2−
7, OsO4) or highly electronegative substances/elements that can gain one or two extra electrons by oxidizing an element or substance (O, F, Cl, Br).Reducers
Substances that have the ability to reduce other substances are said to be reductive or reducing and are known as reducing agents, reductants, or reducers. The reductant (reducing agent) transfers electrons to another substance; i.e., it reduces others, and is thus itself oxidized. And, because it "donates" electrons, it is also called an electron donor. Electron donors can also form charge transfer complexes with electron acceptors.
Reductants in chemistry are very diverse. Electropositive elemental metals, such as lithium, sodium, magnesium, iron, zinc, and aluminium, are good reducing agents. These metals donate or give away electrons readily. Hydride transfer reagents, such as NaBH4 and LiAlH4, are widely used in organic chemistry,[1][2] primarily in the reduction of carbonyl compounds to alcohols. Another method of reduction involves the use of hydrogen gas (H2) with a palladium, platinum, or nickel catalyst. These catalytic reductions are used primarily in the reduction of carbon-carbon double or triple bonds.
Standard Reduction Potential
Reduction potential is used to calculate the standard electrode potential (Eocell).
This is the equation most commonly seen in textbooks:
Eocell = Eored + Eoox .
where: Eocell is the standard electrode potential (in volts).
Eored is standard reduction potential of the reducing agent.
Eoox (standard oxidation potential) is negative of the standard reduction potential of the oxidizing agent.
though the following equation is generally more useful as one is usually given only reduction potentials, not oxidation potentials:
Eocell = Eored - Eoox
or the equivalent:
Eocell = Eocathode - Eoanode
where:
Eocell is the standard electrode potential (in volts).
Eored (Eocathode) is standard reduction potential of the reducing agent.
Eoox (Eoanode) is the standard reduction potential of the oxidizing agent.
Examples of redox reactions
A good example is the reaction between hydrogen and fluorine in which hydrogen is being oxidized and fluorine is being reduced:
- H2 + F2 → 2 HF
We can write this overall reaction as two half-reactions:
the oxidation reaction:
- H2 → 2 H+ + 2 e−
and the reduction reaction:
- F2 + 2 e−
→ 2 F−
Analyzing each half-reaction in isolation can often make the overall chemical process clearer. Because there is no net change in charge during a redox reaction, the number of electrons in excess in the oxidation reaction must equal the number consumed by the reduction reaction (as shown above).
Elements, even in molecular form, always have an oxidation state of zero. In the first half-reaction, hydrogen is oxidized from an oxidation state of zero to an oxidation state of +1. In the second half-reaction, fluorine is reduced from an oxidation state of zero to an oxidation state of −1.
When adding the reactions together the electrons are canceled:
-
H2 → 2 H+ + 2 e− F2 + 2 e− → 2 F− H2 + F2 → 2 H+ + 2 F−
And the ions combine to form hydrogen fluoride:
- H2 + F2 → 2 H+ + 2 F− → 2 HF
Displacement reactions
Redox occurs in single displacement reactions or substitution reactions. The redox component of these types of reactions is the change of oxidation state (charge) on certain atoms, not the actual exchange of atoms in the compounds.
For example, in the reaction between iron and copper(II) sulfate solution:
- Fe + CuSO4 → FeSO4 + Cu
The ionic equation for this reaction is:
- Fe + Cu2+ → Fe2+ + Cu
As two half-equations, it is seen that the iron is oxidized:
- Fe → Fe2+ + 2 e−
And the copper is reduced:
- Cu2+ + 2 e−
→ Cu
Other examples
- The oxidation of iron(II) to iron(III) by hydrogen peroxide in the presence of an acid:
-
- Fe2+ → Fe3+ + e−
- H2O2 + 2 e− → 2 OH−
- Overall equation:
-
- 2 Fe2+ + H2O2 + 2 H+ → 2 Fe3+ + 2 H2O
- The reduction of nitrate to nitrogen in the presence of an acid (denitrification):
-
- 2 NO3− + 10 e− + 12 H+ → N2 + 6 H2O
Iron rusting in pyrite cubes
- Oxidation of elemental iron to iron(III) oxide by oxygen (commonly known as rusting, which is similar to tarnishing):
-
- 4 Fe + 3 O2 → 2 Fe2O3
- The combustion of hydrocarbons, such as in an internal combustion engine, which produces water, carbon dioxide, some partially oxidized forms such as carbon monoxide, and heat energy. Complete oxidation of materials containing carbon produces carbon dioxide.
- In organic chemistry, the stepwise oxidation of a hydrocarbon by oxygen produces water and, successively, an alcohol, an aldehyde or a ketone, a carboxylic acid, and then a peroxide.
Redox reactions in industry
The primary process of reducing ore to produce metals is discussed in the article on Smelting.
Oxidation is used in a wide variety of industries such as in the production of cleaning products and oxidizing ammonia to produce nitric acid, which is used in most fertilizers.
Redox reactions are the foundation of electrochemical cells.
The process of electroplating uses redox reactions to coat objects with a thin layer of a material, as in chrome-plated automotive parts, silver plating cutlery, and gold-plated jewelry.
The production of compact discs depends on a redox reaction, which coats the disc with a thin layer of metal film.[clarification needed]
Redox reactions in biology
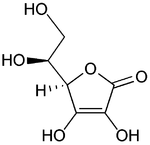
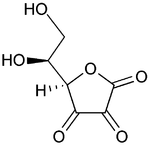 Top: ascorbic acid (reduced form of Vitamin C)
Top: ascorbic acid (reduced form of Vitamin C)
Bottom: dehydroascorbic acid (oxidized form of Vitamin C)Many important biological processes involve redox reactions.
Cellular respiration, for instance, is the oxidation of glucose (C6H12O6) to CO2 and the reduction of oxygen to water. The summary equation for cell respiration is:
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
The process of cell respiration also depends heavily on the reduction of NAD+ to NADH and the reverse reaction (the oxidation of NADH to NAD+). Photosynthesis and Cellular respiration are complementary but photosynthesis is not the reverse of the redox reaction in cell respiration:
- 6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2
Biological energy is frequently stored and released by means of redox reactions. Photosynthesis involves the reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reduce nicotinamide adenine dinucleotide (NAD+), which then contributes to the creation of a proton gradient, which drives the synthesis of adenosine triphosphate (ATP) and is maintained by the reduction of oxygen. In animal cells, mitochondria perform similar functions. See Membrane potential article.
Free radical reactions are redox reactions that occur as a part of homeostasis and killing microorganisms, where an electron detaches from a molecule and then reattaches almost instantaneously. Free radicals are a part of redox molecules and can become harmful to the human body if they do not reattach to the redox molecule or an antioxidant. Unsatisfied free radicals can spur the mutation of cells they encounter and are thus causes of cancer.
The term redox state is often used to describe the balance of NAD+/NADH and NADP+/NADPH in a biological system such as a cell or organ. The redox state is reflected in the balance of several sets of metabolites (e.g., lactate and pyruvate, beta-hydroxybutyrate and acetoacetate), whose interconversion is dependent on these ratios. An abnormal redox state can develop in a variety of deleterious situations, such as hypoxia, shock, and sepsis. Redox signaling involves the control of cellular processes by redox processes.
Redox proteins and their genes must be co-located for redox regulation according to the CoRR hypothesis for the function of DNA in mitochondria and chloroplasts.
Redox cycling
A wide variety of aromatic compounds are enzymatically reduced to form free radicals that contain one more electron than their parent compounds. In general, the electron donor is any of a wide variety of flavoenzymes and their coenzymes. Once formed, these anion free radicals reduce molecular oxygen to superoxide, and regenerate the unchanged parent compound. The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide. This catalytic behavior has been described as futile cycle or redox cycling.
Examples of redox cycling-inducing molecules are the herbicide paraquat and other viologens and quinones such as menadione.[3]
Redox reactions in geology
A uranium mine, near Moab, Utah. Note alternating red and white/green sandstone. This corresponds to oxidized and reduced conditions in groundwater redox chemistry. The rock forms in oxidizing conditions, and is then "bleached" to the white/green state when a reducing fluid passes through the rock. The reduced fluid can also carry uranium-bearing minerals.
In geology, redox is important to both the formation of minerals, mobilization of minerals, and in some depositional environments. In general, the redox state of most rocks can be seen in the color of the rock. Red is associated with oxidizing conditions of formation, and green is typically associated with reducing conditions. White (bleached rock) can also be associated with reducing conditions. Famous examples of redox conditions affecting geological processes include uranium deposits and Moqui marbles.
Balancing redox reactions
Describing the overall electrochemical reaction for a redox process requires a balancing of the component half-reactions for oxidation and reduction. In general, for reactions in aqueous solution, this involves adding H+, OH−, H2O, and electrons to compensate for the oxidation changes.
Acidic media
In acidic media, H+ ions and water are added to half reactions to balance the overall reaction.
For example, when manganese(II) reacts with sodium bismuthate:
-
Unbalanced reaction: Mn2+(aq) + NaBiO3(s) → Bi3+(aq) + MnO4− (aq) Oxidation: 4 H2O(l) + Mn2+(aq) → MnO−
4(aq) + 8 H+(aq) + 5 e−Reduction: 2 e−
+ 6 H+ + BiO−
3(s) → Bi3+(aq) + 3 H2O(l)
The reaction is balanced by scaling the two half-cell reactions to involve the same number of electrons (multiplying the oxidation reaction by the number of electrons in the reduction step and vice versa):
- 8 H2O(l) + 2 Mn2+(aq) → 2 MnO−
4(aq) + 16 H+(aq) + 10 e− - 10 e−
+ 30 H+ + 5 BiO−
3(s) → 5 Bi3+(aq) + 15 H2O(l)
Adding these two reactions eliminates the electrons terms and yields the balanced reaction:
- 14 H+(aq) + 2 Mn2+(aq) + 5 NaBiO3(s) → 7 H2O(l) + 2 MnO−
4(aq) + 5 Bi3+(aq) + 5 Na+(aq)
Basic media
In basic media, OH− ions and water are added to half reactions to balance the overall reaction.
For example, in the reaction between potassium permanganate and sodium sulfite:
-
Unbalanced reaction: KMnO4 + Na2SO3 + H2O → MnO2 + Na2SO4 + KOH Reduction: 3 e−
+ 2 H2O + MnO4− → MnO2 + 4 OH−Oxidation: 2 OH− + SO32− → SO42− + H2O + 2 e−
Balancing the number of electrons in the two half-cell reactions gives:
- 6 e−
+ 4 H2O + 2 MnO4− → 2 MnO2 + 8 OH− - 6 OH− + 3 SO32− → 3 SO42− + 3 H2O + 6 e−
Adding these two half-cell reactions together gives the balanced equation:
- 2 KMnO4 + 3 Na2SO3 + H2O → 2 MnO2 + 3 Na2SO4 + 2 KOH
Memory aids
The key terms involved in redox are often confusing to students.[4][5] For example, an element that is oxidized loses electrons; however, that element is referred to as the reducing agent. Likewise, an element that is reduced gains electrons and is referred to as the oxidizing agent.[6] Acronyms or mnemonics are commonly used[7] to help remember what is happening:
- "LEO the lion says GER" — Loss of Electrons is Oxidation, Gain of Electrons is Reduction.[4][5][7][6]
- "LEORA says GEROA" — Loss of Electrons is Oxidation (Reducing Agent) and Gain of Electrons is Reduced (Oxidizing Agent).[6]
See also
References
- ^ Hudlický, Miloš (1996). Reductions in Organic Chemistry. Washington, D.C.: American Chemical Society. pp. 429. ISBN 0-8412-3344-6.
- ^ Hudlický, Miloš (1990). Oxidations in Organic Chemistry. Washington, D.C.: American Chemical Society. pp. 456. ISBN 0-8412-1780-7.
- ^ "gutier.doc". http://www.bioscience.org/2000/v5/d/gutier/gutier.pdf. Retrieved 2008-06-30.PDF (2.76 MiB)
- ^ a b c Robertson, William (2010). More Chemistry Basics. National Science Teachers Association. p. 82. ISBN 978-1-936137-74-9. http://books.google.com/books?id=hIzuarlXtH4C&pg=PA82&dq=confusing.
- ^ a b c Phillips, John; Strozak, Victor; Wistrom, Cheryl (2000). Chemistry: Concepts and Applications. Glencoe McGraw-Hill. p. 558. ISBN 978-0028282107. "Students often are confused when associating reduction with the gain of electrons."
- ^ a b c d Rodgers, Glen (2012). Descriptive Inorganic, Coordination, and Solid-State Chemistry. Brooks/Cole, Cengage Learning. p. 330. ISBN 978-0-8400-6846-0. http://books.google.com/books?id=g_ybia0hGw8C&pg=PA330.
- ^ a b c Zumdahl, Steven; Zumdahl, Susan (2009). Chemistry. Houghton Mifflin. p. 160. ISBN 978-0-547-05405-6. http://books.google.com/books?id=IdhYqXy37KIC&pg=PA160.
- Schüring, J., Schulz, H. D., Fischer, W. R., Böttcher, J., Duijnisveld, W. H. (editors)(1999). Redox: Fundamentals, Processes and Applications, Springer-Verlag, Heidelberg, 246 pp. ISBN 978-3-540-66528-1 (pdf 3,6 MB)
External links
- Chemical Equation Balancer - An open source chemical equation balancer that handles redox reactions.
- Video - Synthesis of Copper(II) Acetate 20 Feb. 2009
- Redox reactions calculator
- Redox reactions at Chemguide
- Online redox reaction equation balancer, balances equations of any half-cell and full reactions
Categories:- Soil chemistry
- Chemical reactions
Wikimedia Foundation. 2010.





