- Denitrification
-
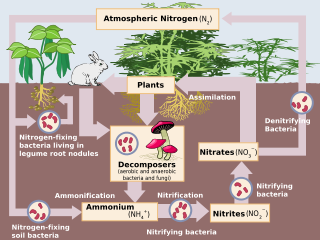
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products.
This respiratory process reduces oxidized forms of nitrogen in response to the oxidation of an electron donor such as organic matter. The preferred nitrogen electron acceptors in order of most to least thermodynamically favorable include nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), and nitrous oxide (N2O). In terms of the general nitrogen cycle, denitrification completes the cycle by returning N2 to the atmosphere.
The process is performed primarily by heterotrophic bacteria (such as Paracoccus denitrificans and various pseudomonads),[1] although autotrophic denitrifiers have also been identified (e.g., Thiobacillus denitrificans).[2] Denitrifiers are represented in all main phylogenetic groups.[3] Generally several species of bacteria are involved in the complete reduction of nitrate to molecular nitrogen, and more than one enzymatic pathway have been identified in the reduction process.[4]
Direct reduction from nitrate to ammonium, a process known as dissimilatory nitrate reduction to ammonium or DNRA,[5] is also possible for organisms that have the nrf-gene.[6] This is less common than denitrification in most ecosystems as a means of nitrate reduction. Other genes known in microorganisms which denitrify include nir (nitrite reductase) and nos (nitrous oxide reductase) among others;[7] organisms identified as having these genes include Alcaligenes faecalis, Alcaligenes xylosoxidans, many in the Pseudomonas genus, Bradyrhizobium japonicum, and Blastobacter denitrificans.[8]
Contents
Nutrient limitation
All organisms require certain nutrients in their surroundings (available to them) for survival.[9] Depending upon the ecosystem, nitrogen is most likely the limiting nutrient, although phosphorus is the other primary limiting nutrient and these two elements interact chemically.[10] Some organisms appear to be able to denitrify and remove phosphorus.[11] The triple bond of N2 makes this a very stable compound; most organisms (i.e. plants) depend upon others to break this down to make it available for biochemical reactions.[12] See Nitrification. Symbiotic relationships between Rhizobium species and legumes are well-documented.[13]
Conditions required
Denitrification takes place under special conditions in both terrestrial and marine ecosystems.[14] In general, it occurs where oxygen, a more energetically favourable electron acceptor, is depleted, and bacteria respire nitrate as a substitute terminal electron acceptor. Due to the high concentration of oxygen in our atmosphere denitrification only takes place in anaerobic environments where oxygen consumption exceeds the oxygen supply and where sufficient quantities of nitrate are present. These environments may include certain soils[15] and groundwater,[16] wetlands, oil reservoirs,[17] poorly ventilated corners of the ocean, and in seafloor sediments.
Denitrification generally proceeds through some combination of the following intermediate forms:
- NO3− → NO2− → NO + N2O → N2 (g)
The complete denitrification process can be expressed as a redox reaction:
- 2 NO3− + 10 e− + 12 H+ → N2 + 6 H2O
This reaction shows a fractionation in isotope composition. Lighter isotopes of nitrogen are preferred in the reaction, leaving the heavier nitrogen isotopes in the residual matter. The process can cause delta-values of up to −40, where delta is a representation of the difference in isotopic composition. This can be used to identify denitrification processes in nature.
Denitrification by rhizobia
Rhizobia are soil bacteria with the unique ability to establish a N2-fixing symbiosis on legume roots. When faced with a shortage of oxygen some rhizobia species are able to switch from O2-respiration to using nitrates to support respiration. This denitrification pathway comprises the sequential reduction of nitrate or nitrite to dinitrogen, via the gaseous intermediates nitric oxide and nitrous oxide. The enzymes involved in denitrification are nitrate-, nitrite-, nitric oxide- and nitrous oxide reductase, encoded by nar/nap, nir, nor and nos genes, respectively. In recent years it has emerged that many rhizobia species have genes for enzymes of some or all of the four reductase reactions for denitrification. In fact, denitrification can be readily observed in many rhizobia species, in their free-living form, in legume root nodules, or in isolated bacteroids.[18]
Deliberate use of process
Denitrification is commonly used to remove nitrogen from sewage and municipal wastewater. It is also an instrumental process in wetlands[19] and riparian zones[20] for the removal of excess nitrate from groundwater resulting from excessive agricultural or residential fertilizer usage.[21]
Reduction under anoxic conditions can also occur through process called anaerobic ammonium oxidation (anammox):[22]
- NH4+ + NO2− → N2 + 2 H2O
In some wastewater treatment plants, small amounts of methanol, ethanol, acetate or proprietary products like MicroCg or MicroCglycerin are added to the wastewater to provide a carbon source for the denitrification bacteria.[23] Denitrification processes are also used in the treatment of industrial wastes.[24]
Influence on global climate change
Increasing carbon dioxide levels within the atmosphere will influence global nutrient cycling, yet it is difficult to predict what those interactions might be.[25] Chemical interactions between soils and the atmosphere will be influenced by changes in atmospheric composition.[26] There are indications that increased fertilization of soils with nitrogen causes a decrease in carbon sequestration.[27]
Jake Beaulieu, a postdoctoral researcher the Environmental Protection Agency in Cincinnati, Ohio and Jennifer Tank, Galla Professor of Biological Sciences at the University of Notre Dame, are lead authors of new paper demonstrating that streams and rivers receiving nitrogen inputs from urban and agricultural land uses are a significant source of nitrous oxide to the atmosphere.[28]
See also
- Aerobic denitrification
- Anaerobic respiration
- Anammox
- Bioremediation
- Climate change
- Hypoxia (environmental)
- Nitrogen cycle
- Sewage treatment
- Simultaneous nitrification-denitrification
- Nitrogen fixation
References
- ^ Carlson, C. A., and J. L. Ingraham. 1983. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl. Environ. Microbiol. 45:1247-1253.
- ^ Baalsrud, K., and K. S. Baalsrud. 1954. Studies on Thiobacillus denitrificans. Archives of Microbiology 20:34-62.
- ^ Zumft, W. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616.
- ^ Atlas, R.M., Barthas, R. Microbial Ecology: Fundamentals and Applications. 3rd Ed. Benjamin-Cummings Publishing. ISBN 0805306536
- ^ An, S., and W. S. Gardner. 2002. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Marine Ecology Progress Series 237:41-50.
- ^ Spanning, R., M. Delgado, and D. Richardson. 2005. "It is possible to encounter DNRA when your source of carbon is a fermentable substrate, as glucose, so if you wanna avoid DNRA use a non fermentable substrate. The Nitrogen Cycle: Denitrification and its Relationship to N2 Fixation, p. 277-342."
- ^ Zumft, W. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616.
- ^ Liu, X., S. M. Tiquia, G. Holguin, L. Wu, S. C. Nold, A. H. Devol, K. Luo, A. V. Palumbo, J. M. Tiedje, and J. Zhou. 2003. Molecular Diversity of Denitrifying Genes in Continental Margin Sediments within the Oxygen-Deficient Zone off the Pacific Coast of Mexico. Appl. Environ. Microbiol. 69:3549-3560
- ^ Howarth, R. W. 1988. Nutrient Limitation of Net Primary Production in Marine Ecosystems. Annual Review of Ecology and Systematics 19:89-110.
- ^ Vance, C. P. 2001. Symbiotic Nitrogen Fixation and Phosphorus Acquisition. Plant Nutrition in a World of Declining Renewable Resources. Plant Physiol. 127:390-397.
- ^ Kuba, T., M. C. M. Van Loosdrecht, F. A. Brandse, and J. J. Heijnen. 1997. Occurrence of denitrifying phosphorus removing bacteria in modified UCT-type wastewater treatment plants. Water Research 31:777-786.
- ^ Seitzinger, S., J. A. Harrison, J. K. Bohlke, A. F. Bouwman, R. Lowrance, B. Peterson, C. Tobias, and G. V. Drecht. 2006. Denitrification Across Landscapes and Waterscapes: A Synthesis. Ecological Applications 16:2064-2090.
- ^ Daniel, R. M., I. M. Smith, J. A. D. Phillip, H. D. Ratcliffe, J. W. Drozd, and A. T. Bull. 1980. Anaerobic Growth and Denitrification by Rhizobium japonicum and Other Rhizobia. J Gen Microbiol 120:517-521.
- ^ Seitzinger, S., J. A. Harrison, J. K. Bohlke, A. F. Bouwman, R. Lowrance, B. Peterson, C. Tobias, and G. V. Drecht. 2006. Denitrification Across Landscapes and Waterscapes: A Synthesis. Ecological Applications 16:2064-2090.
- ^ Scaglia, J.; Lensi, R.; Chalamet, A. (1985). "Relationship between photosynthesis and denitrification in planted soil". Plant and Soil 84 (1): 37–43. doi:10.1007/BF02197865.
- ^ Korom, Scott F. (1992). "Natural Denitrification in the Saturated Zone: A Review". Water Resources Research 28 (6): 1657–1668. Bibcode 1992WRR....28.1657K. doi:10.1029/92WR00252.
- ^ Cornish Shartau, S. L.; Yurkiw, M.; Lin, S.; Grigoryan, A. A.; Lambo, A.; Park, H-S.; Lomans, B. P.; Van Der Biezen, E. et al. (2010). "Ammonium Concentrations in Produced Waters from a Mesothermic Oil Field Subjected to Nitrate Injection Decrease through Formation of Denitrifying Biomass and Anammox Activity". Applied and Environmental Microbiology 76 (15): 4977–4987. doi:10.1128/AEM.00596-10. PMC 2916462. PMID 20562276. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2916462.
- ^ Moir, JWB (editor) (2011). Nitrogen Cycling in Bacteria: Molecular Analysis. Caister Academic Press. ISBN 978-1-904455-86-8.
- ^ Bachand, P. A. M., and A. J. Horne. 1999. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecological Engineering 14:17-32.
- ^ Martin, T. L., N. K. Kaushik, J. T. Trevors, and H. R. Whiteley. 1999. Review: Denitrification in temperate climate riparian zones. Water, Air, and Soil Pollution 111:171-186.
- ^ Mulvaney, R. L., S. A. Khan, and C. S. Mulvaney. 1997. Nitrogen fertilizers promote denitrification. Biology and Fertility of Soils 24:211-220.
- ^ Dalsgaard, T., B. Thamdrup, and D. E. Canfield. 2005. Anaerobic ammonium oxidation (anammox) in the marine environment. Research in Microbiology 156:457-464.
- ^ Chen, K.-C., and Y.-F. Lin. 1993. The relationship between denitrifying bacteria and methanogenic bacteria in a mixed culture system of acclimated sludges. Water Research 27:1749-1759.
- ^ Constantin, H., and M. Fick. 1997. Influence of C-sources on the denitrification rate of a high-nitrate concentrated industrial wastewater. Water Research 31:583-589.
- ^ Sinclair, T. R. 1992. Mineral Nutrition and Plant Growth Response to Climate Change. J. Exp. Bot. 43:1141-1146.
- ^ Rosenzweig, C., and D. Hillel. 2000. Soils and Global Climate Change: Challenges and Opportunities. Soil Science 165:47-56.
- ^ Oren, R., D. S. Ellsworth, K. H. Johnsen, N. Phillips, B. E. Ewers, C. Maier, K. V. R. Schafer, H. McCarthy, G. Hendrey, S. G. McNulty, and G. G. Katul. 2001. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469-472.
- ^ http://www.eurekalert.org/pub_releases/2010-12/uond-nsf122010.php
Literature
Atlas, R.M., Barthas, R. Microbial Ecology: Fundamentals and Applications. 3rd Ed. Benjamin-Cummings Publishing. ISBN 0805306536
Zumft, W.G. (1997): Cell biology and molecular basis of denitrification. In: Microbiol. Mol. Biol. Rev. Bd. 61, Nr. 4, S. 533-616. PMID 9409151 PDF
Categories:- Nitrogen metabolism
- Environmental microbiology
Wikimedia Foundation. 2010.