- Permanganate
-
A permanganate is the general name for a chemical compound containing the manganate(VII) ion, (MnO4−). Because manganese is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion has tetrahedral geometry.[1] Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media.
In an acidic solution, permanganate(VII) is reduced to the colourless +2 oxidation state of the manganese(II) (Mn2+) ion.
- 8 H+ + MnO4− + 5 e− → Mn2+ + 4 H2O
In a strongly basic solution, permanganate(VII) is reduced to the green +6 oxidation state of the manganate MnO42−.
- MnO4− + e− → MnO42−
In a neutral medium however, it gets reduced to the brown +4 oxidation state of manganese dioxide MnO2.
- 2 H2O + MnO4− + 3 e− → MnO2 + 4 OH−
Contents
Production
Permanganates can be produced by oxidation of manganese compounds such as manganese chloride or manganese sulfate by strong oxidizing agents, for instance, sodium hypochlorite or lead dioxide:
- 2 MnCl2 + 5 NaClO + 6 NaOH → 2 NaMnO4 + 9 NaCl+ 3 H2O
- 2 MnSO4 + 5 PbO2+ 3 H2SO4 → 2 HMnO4 + 5 PbSO4 + 2 H2O
It may also be produced by the dismutation of manganates, with manganese dioxide as a side-product:
- 3 Na2MnO4 + 2 H2O → 2 NaMnO4 + MnO2 + 4 NaOH
Properties
Permanganates(VII) are salts of permanganic acid. Permanganate(VII) is a strong oxidizer, and similar to perchlorate. It is therefore in common use in qualitative analysis that involves redox reactions (permanganometry). Besides this, it is stable.
It is a useful reagent, though with organic compounds, not very selective.
Manganates(VII) are not very stable thermally. For instance, potassium permanganate decomposes at 230 °C to potassium manganate and manganese dioxide, releasing oxygen gas:
- 2 KMnO4 → K2MnO4 + MnO2 + O2
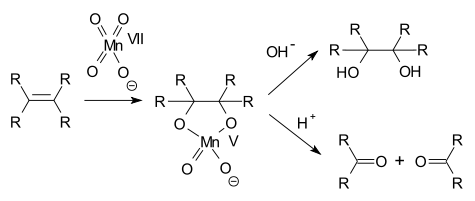
A permanganate can oxidize an amine to a nitro compound,[2][3] an alcohol to a ketone,[4] an aldehyde to a carboxylic acid,[5][6] a terminal alkene to a carboxylic acid,[7] oxalic acid to carbon dioxide,[8] and an alkene to a diol.[9] This list not exhaustive.
In alkene oxidations one intermediate is a cyclic Mn(V) species:
Compounds
- Ammonium permanganate, NH4MnO4
- Calcium permanganate, Ca(MnO4)2
- Potassium permanganate, KMnO4
- Sodium permanganate, NaMnO4
See also
- Perchlorate, a similar ion with a chlorine(VII) center
- Chromate, which is isoelectronic with permanganate
References
- ^ Sukalyan Dash, Sabita Patel and Bijay K. Mishra (2009). "Oxidation by permanganate: synthetic and mechanistic aspects". Tetrahedron 65 (4): 707–739. doi:10.1016/j.tet.2008.10.038.
- ^ A. Calder, A. R. Forrester1, and S. P. Hepburn (1972), "2-methyl-2-nitrosopropane and its dimer", Org. Synth. 6: 803, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV6P0803; Coll. Vol. 52: 77
- ^ Nathan Kornblum and Willard J. Jones (1963), "4-nitro-2,2,4-trimethylpentane", Org. Synth. 5: 845, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV5P0845; Coll. Vol. 43: 87
- ^ J. W. Cornforth (1951), "Ethyl pyruvate", Org. Synth. 4: 467, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV4P0467; Coll. Vol. 31: 59
- ^ R. L. Shriner and E. C. Kleiderer (1930), "Piperonylic acid", Org. Synth. 2: 538, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0538; Coll. Vol. 10: 82
- ^ John R. Ruhoff (1936), "n-heptanoic acid", Org. Synth. 2: 315, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0315; Coll. Vol. 16: 39
- ^ Donald G. Lee, Shannon E. Lamb, and Victor S. Chang (1981), "Carboxylic acids from the oxidation of terminal alkenes by permanganate: nonadecanoic acid", Org. Synth. 7: 397, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV7P0397; Coll. Vol. 60: 11
- ^ Kovacs KA, Grof P, Burai L, Riedel M (2004). "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A 108 (50): 11026. doi:10.1021/jp047061u.
- ^ E. J. Witzemann, Wm. Lloyd Evans, Henry Hass, and E. F. Schroeder (1931), "dl-glyceraldehyde ethyl acetal", Org. Synth. 2: 307, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=CV2P0307; Coll. Vol. 11: 52
Categories:- Oxoanions
Wikimedia Foundation. 2010.