- Potassium permanganate
-
Potassium permanganate 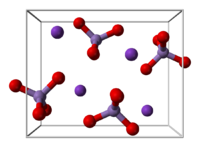
 Potassium manganate(VII)Other namesPotassium permanganate
Potassium manganate(VII)Other namesPotassium permanganate
Potassium manganate(VII)
Chameleon mineral
Condy's crystals
Permanganate of potashIdentifiers CAS number 7722-64-7 
PubChem 24400 ChemSpider 22810 
EC number 231-760-3 UN number 1490 KEGG D02053 
RTECS number SD6475000 Jmol-3D images Image 1 - [K+].[O-][Mn](=O)(=O)=O
Properties Molecular formula KMnO4 Molar mass 158.034 g/mol Appearance purplish-bronze-gray needles
magenta–rose in solutionOdor odorless Density 2.703 g/cm3 Melting point 240 °C, 513 K, 464 °F (decomp.)
Solubility in water 6.38 g/100 mL (20 °C)
25 g/100 mL (65 °C)Solubility decomposes in alcohol and organic solvents Structure Crystal structure Orthorhombic Thermochemistry Std enthalpy of
formation ΔfHo298−813.4 kJ/mol Standard molar
entropy So298171.7 J K–1 mol–1 Hazards MSDS External MSDS EU Index 025-002-00-9 EU classification Oxidant (O)
Harmful (Xn)
Dangerous for the environment (N)
Non-FlammableR-phrases R8, R22, R50/53 S-phrases (S2), S60, S61 NFPA 704 Related compounds Other anions Potassium manganite
Potassium manganateOther cations Sodium permanganate
Ammonium permanganateRelated compounds Manganese heptoxide  permanganate (verify) (what is:
permanganate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the evaporation of which leaves prismatic purplish-black glistening crystals.[1] In 2000, worldwide production was estimated at 30,000 tonnes.[2] In this compound, manganese is in the +7 oxidation state.
Contents
Structure and preparation
Potassium permanganate is produced industrially from manganese dioxide, which also occurs as the mineral pyrolusite. The MnO2 is fused with potassium hydroxide and heated in air or with a source of oxygen, like potassium nitrate or chlorate.[2] This process gives potassium manganate, which upon electrolytic oxidation in alkaline media, or by boiling the manganate solution in the presence of carbon dioxide until all the green color is discharged, gives potassium permanganate[3].
- 2 MnO2 + 4 KOH + O2 → 2 K2MnO4 + 2 H2O
- 2 MnO42– + Cl2 → 2 MnO4– + 2 Cl–
or:
- 3 K2MnO4 + 2 CO2 → 2 KMnO4 + 2 K2CO3 + MnO2
In which the potassium permanganate is separated by filtering the insoluble manganese dioxide, evaporating the solution to 1/3 and recrystallizing it.
Permanganate salts can also be generated by treating a solution of Mn2+ ions with strong oxidants such as lead dioxide (PbO2), or sodium bismuthate (NaBiO3). Tests for the presence of manganese exploit the vivid violet colour of permanganate produced by these reagents.
KMnO4 forms orthorhombic crystals with constants: a = 910.5 pm, b = 572.0 pm, c = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions.[4] In the solid (as in solution), each MnO4- centres are tetrahedral. The Mn-O distances are 1.62 Å.[5]
Reactions
 A solution of KMnO4 in water, in a volumetric flask
A solution of KMnO4 in water, in a volumetric flask
Analytical
Potassium permanganate can be used to quantitatively determine the total oxidisable organic material in an aqueous sample. The value determined is known as the permanganate value. In analytical chemistry, a standardized aqueous solution of KMnO4 is sometimes used as an oxidizing titrant for redox titrations (permanganometry). In a related way, it is used as a reagent to determine the Kappa number of wood pulp. For the standardization of KMnO4 solutions, reduction by oxalic acid is often used.[6]
Aqueous, acidic solutions of KMnO4 are used to collect gaseous mercury in flue gas during stationary source emissions testing.[7]
Organic chemistry
Dilute solutions of KMnO4 convert alkenes into diols (glycols). This behaviour is also used as a qualitative test for the presence of double or triple bonds in a molecule, since the reaction decolourises the permanganate solution. It is sometimes referred to as Baeyer's reagent. However, bromine serves better in measuring unsaturation (double or triple bonds) quantitatively, since KMnO4, being a very strong oxidizing agent, can react with a variety of groups.
Under acidic conditions, the alkene double bond is cleaved to give the appropriate carboxylic acid:[8]
- CH3(CH2)17CH=CH2 + 2 KMnO4 + 3 H2SO4 → CH3(CH2)17COOH + CO2 + 4 H2O + K2SO4 + 2 MnSO4
Potassium permanganate oxidizes aldehydes to carboxylic acids, such as the conversion of n-heptanal to heptanoic acid:[9]
- 5 C6H13CHO + 2 KMnO4 + 3 H2SO4 → 5 C6H13COOH + 3 H2O + K2SO4 + 2 MnSO4
Even an alkyl group (with a benzylic hydrogen) on an aromatic ring is oxidized, e.g. toluene to benzoic acid.[10]
- 5 C6H5CH3 + 6 KMnO4 + 9 H2SO4 → 5 C6H5COOH + 14 H2O + 2 K2SO4 + 6 MnSO4
Glycols and polyols are highly reactive toward KMnO4. For example, addition of potassium permanganate to an aqueous solution of sugar and sodium hydroxide produces the "chemical chameleon" reaction, which involves dramatic colour changes associated with the various oxidation states of manganese. A related vigorous reaction is exploited as a fire starter in survival kits. For example, a mixture of potassium permanganate and glycerol or pulverized glucose ignites readily.[11] Its sterilizing properties are another reason for inclusion of KMnO4 in a survival kit.
"Purple benzene"
Purple benzene refers to solutions prepared by treating a two phase mixture of aqueous potassium permanganate and benzene with a quat salt. The quat cation forms a salt of permanganate that is soluble in benzene, giving this organic solvent a purple color. The reaction illustrate the ability of quat salts to confer lipophilicity to hydrophilic anions. The same effect can be obtained by complexing the potassium to 18-crown-6.[12]
Reaction with acids
Concentrated sulfuric acid reacts with KMnO4 to give Mn2O7, which can be explosive.[13] Similarly concentrated hydrochloric acid gives chlorine. The Mn-containing products from redox reactions depend on the pH. Acidic solutions of permanganate are reduced to the faintly pink manganese(II) ion (Mn2+) and water. In neutral solution, permanganate is only reduced by 3e− to give MnO2, wherein Mn is in a +4 oxidation state. This is the material that stains one's skin when handling KMnO4. KMnO4 spontaneously reduces in an alkaline solution to green K2MnO4, wherein manganese is in the +6 oxidation state.
A curious reaction occurs upon addition of concentrated sulfuric acid to potassium permanganate. Although no reaction may be apparent, the vapor over the mixture will ignite paper impregnated with alcohol. Potassium permanganate and sulfuric acid react to produce some ozone, which has a high oxidising power and rapidly oxidises the alcohol, causing it to combust. As the reaction also produces explosive Mn2O7, this should only be attempted with great care.[14][15]
Photodecomposition
Potassium permanganate decomposes when exposed to light:
- 2 KMnO4 → K2MnO4 + MnO2(s)+O2
Handling
As an oxidizer that generates the dark brown product MnO2, potassium permanganate rapidly stains virtually any organic material such as skin, paper, and clothing. This staining effect is used to "develop" TLC plates. The redox reaction is used for artistic purposes as an agent to prepare paper for fast bleaching. Lemon juice is enough to quickly remove colour from the paper and applied with a paint brush this can create interesting aesthetics.[citation needed] Even glassware containing solutions of KMnO4 can become brown. MnO2 can be removed by scrubbing with dilute acids ( a lemon rubbed into the skin works especially well) or with sodium thiosulfate.
Uses
Almost all applications of potassium permanganate exploit its oxidizing properties.[2] As a strong oxidant that does not generate toxic byproducts, KMnO4 has many niche uses.
Potassium permanganate is one of the principal chemicals used in the film and television industries to "age" props and set dressings. Its oxidising effects create "hundred year old" or "ancient" looks on hessian cloth, ropes, timber and glass.[16] It was used on props and sets in films such as "Troy", "300" and "Indiana Jones".[citation needed]
Disinfectant and water treatment
As an oxidant, potassium permanganate can act as an antiseptic. For example, dilute solutions are used to treat canker sores (ulcers), disinfectant for the hands and treatment for mild pompholyx, dermatitis,[17][18] and fungal infections of the hands or feet.[19] Potassium permanganate is used extensively in the water treatment industry. It is used as a regeneration chemical to remove iron and hydrogen sulfide (rotten egg smell) from well water via a "Manganese Greensand" Filter. "Pot-Perm" is also obtainable at pool supply stores, is used additionally to treat waste water. Historically it was used to disinfect drinking water.[20][21] It currently finds application in the control of nuisance organisms such as Zebra mussels in fresh water collection and treatment systems.[22]
Biomedical uses
Related to the use of KMnO4 for water treatment, this salt is often employed as a specialized disinfectant for treating human and animal ailments. In histology, it is used to bleach melanin which obscures tissue detail. Potassium permanganate can also be used to differentiate amyloid AA from other types of amyloid pathologically deposited in body tissues. Incubation of fixed tissue with potassium permanganate will prevent amyloid AA from staining with congo red whereas other types of amyloid are unaffected.[23][24] Permanganate washes were once used to treat gonorrhea[25] and are still used to treat candidiasis.[26] It can be used to inactivate the poison strychnine.
Organic synthesis
Aside from its use in water treatment, the other major application of KMnO4 is as a reagent for the synthesis of organic compounds.[27] Significant amounts are required for the synthesis of ascorbic acid, chloramphenicol, saccharin, isonicotinic acid, and pyrazinoic acid.[2]
Fruit preservation
Addition of potassium permanganate substrate to banana shipments adsorbs the ethylene and doubles the fruit ripening period (see banana entry for more details).
Survival kits
Potassium permanganate is typically included in survival kits: as a fire starter,[11] water sterilizer and for creating distress signals on snow.[28]
History
In 1659, Johann Rudolf Glauber fused a mixture of the mineral pyrolusite and potassium carbonate to obtain a material that, when dissolved in water, gave a green solution (potassium manganate) which slowly shifted to violet and then finally red. This report represents the first description of the production of potassium permanganate.[29] Just under two hundred years later London chemist Henry Bollmann Condy had an interest in disinfectants, and marketed several products including ozonised water. He found that fusing pyrolusite with NaOH and dissolving it in water produced a solution with disinfectant properties. He patented this solution, and marketed it as Condy's Fluid. Although effective, the solution was not very stable. This was overcome by using KOH rather than NaOH. This was more stable, and had the advantage of easy conversion to the equally effective potassium permanganate crystals. This crystalline material was known as Condy's crystals or Condy's powder. Potassium permanganate was comparatively easy to manufacture so Condy was subsequently forced to spend considerable time in litigation in order to stop competitors from marketing products similar to Condy's Fluid or Condy's Crystals.[30]
Early photographers used it as a component of flash powder. It is now replaced with other oxidizers, due to the instability of permanganate mixtures. Aqueous solutions of KMnO4 have been used together with T-Stoff (i.e. 80% hydrogen peroxide) as propellant for the rocket plane Messerschmitt Me 163. In this application, it was known as Z-Stoff. This combination of propellants is sometimes still used in torpedoes.
Safety
Solid KMnO4 is a strong oxidizer and thus should be kept separated from oxidizable substances. Reaction with concentrated sulfuric acid produces the highly explosive manganese(VII) oxide (Mn2O7). When solid KMnO4 is mixed with pure glycerol or other simple alcohols it will result in a violent combustion reaction.
Potassium permanganate may leave behind a brownish stain which can be removed by using sodium bisulfite or oxalic acid.
References
- ^ F. Burriel, F. Lucena, S. Arribas and J. Hernández, (1985), Química Analítica Cualitativa, page 688, ISBN 84-9732-140-5.
- ^ a b c d Arno H. Reidies "Manganese Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_123
- ^ H. F. Walton. Inorganic Preparations. New York, 1948.
- ^ "Handbook of Preparative Inorganic Chemistry"; Brauer, E., Ed.; Academic: New York, 1963
- ^ Gus J. Palenik "Crystal structure of potassium permanganate" Inorg. Chem., 1967, volume 6, pp 503–507.doi:10.1021/ic50049a015
- ^ Kovacs KA, Grof P, Burai L, Riedel M (2004). "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A 108 (50): 11026. doi:10.1021/jp047061u.
- ^ Code of Federal Regulations(7-1-07) Edition, Title 40, Part 60, Appendix A-8, Method 29, Section 7.3.1
- ^ Donald G. Lee, Shannon E. Lamb, and Victor S. Chang (1990), "Carboxylic Acids from the Oxidation of Terminal Alkenes by Permanganate: Nonadecanoic Acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv7p0397; Coll. Vol. 7: 397
- ^ John R. Ruhoff, "n-Heptanoic acid", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0315; Coll. Vol. 2: 315
- ^ Gardner KA, Mayer JM (1995). "Understanding C-H Bond Oxidations: H· and H- Transfer in the Oxidation of Toluene by Permanganate". Science 269 (5232): 1849. doi:10.1126/science.7569922. PMID 7569922.
- ^ a b Bob Gillis and Dino Labiste. "Chemical Fire". http://www.primitiveways.com/chemical_fire.html.
- ^ Anthony J. Doheny Jr. and Bruce Ganem "Purple benzene revisited" J. Chem. Educ., 1980, volume 57, p 308. doi:10.1021/ed057p308.1
- ^ F. A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann (April 1999). Advanced Inorganic Chemistry, 6th Edition. Wiley-VCH. ISBN 0-471-19957-5
- ^ Barthel, H. and Duvinage, B. (2000). "Clemens Winkler. His Experiments with Ozone in 1892". Praxis der Naturwissenschaften, Chemie 49: 18ff.
- ^ Dzhabiev, T. S.; Denisov, N. N.; Moiseev, D. N. and Shilov, A. E. (2005). "Formation of Ozone During the Reduction of Potassium Permanganate in Sulfuric Acid Solutions". Russian Journal of Physical Chemistry 79: 1755–1760.
- ^ Ester Brody (February 2000). "Victor DeLor contractor profile". PaintPRO 2 (1). http://www.paintpro.net/Articles/PP201/PP201-Contractor_Profile.cfm. Retrieved 2009-11-12. "One of the techniques DeLor is known for among designers and clients is the special effects he creates with various chemical solutions. When applied to wood surfaces, these chemicals give a weathered appearance to new wood. ... To achieve the aesthetic on interior surfaces, DeLor often uses a mixture of water and potassium permanganate, a dry powder chemical."
- ^ BIRT AR (March 1964). "Drugs for eczema of children". Can Med Assoc J 90 (11): 693–4. PMC 1922428. PMID 14127384. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1922428.
- ^ Stalder JF, Fleury M, Sourisse M, et al. (1992). "Comparative effects of two topical antiseptics (chlorhexidine vs KMn04) on bacterial skin flora in atopic dermatitis". Acta Derm Venereol Suppl (Stockh) 176: 132–4. PMID 1476027.
- ^ Program for Appropriate Technology in Health PATH (1988). "Skin diseases". Health Technol Dir 8 (3): 1–10. PMID 12282068.
- ^ Assembly of Life Sciences (U.S.). Safe Drinking Water Committee (1977). Drinking water and health, Volume 2. National Academies Press. p. 98. ISBN 9780309029315. http://books.google.com/?id=uVlQ_TUP-f0C&pg=PA98I.
- ^ "Red faces over pink water", The Northern Advocate
- ^ EPA Guidance Manual Alternative Disinfectants and Oxidants
- ^ Wright, J. R.; Calkins E.; Humphrey R. L. (1977). "Potassium permanganate reaction in amyloidosis. A histologic method to assist in differentiating forms of this disease". Lab Invest. 36 (3): 274–81. PMID 839739.
- ^ van Rijswijk, M. H.; van Heusden, C. W. (1979). "The potassium permanganate method. A reliable method for differentiating amyloid AA from other forms of amyloid in routine laboratory practice". Am J Pathol 97 (1): 43–58. PMC 2042379. PMID 495695. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2042379.
- ^ "Safe Sex Campaigner", Ettie Rout at archives.govt.nz
- ^ Haseltine, Florence; Yvonne Yaw (1976). "Private Medicine". Woman Doctor: the Internship of a Modern Woman (Book Club ed.). Houghton Mifflin. p. 32.
- ^ A. Fatiadi (1987). "The Classical Permanganate Ion: Still a Novel Oxidant in Organic Chemistry" (review). Synthesis 1987 (2): 85–127. doi:10.1055/s-1987-27859.
- ^ "Distress Signals", Evening Post, Volume CXXI, Issue 107, 7 May 1936, Page 5
- ^ Weeks, M. E. and Leicester, H. M.; Discovery of the Elements, Journal of Chemical Education 1968
- ^ "Important Trade Mark Case", Otago Witness, Volume 02, Issue 2420, 2 August 1900, Page 53
External links
- International Chemical Safety Card 0672
- National Pollutant Inventory: Manganese and compounds Fact Sheet
- The use of potassium permanganate in fish ponds
- Sugar, NaOH and KMnO4
Manganese compounds Potassium compounds KBr · KBrO3 · KCN · KCNO · KCl · KClO3 · KClO4 · KF · KH · KHCO2 · KHCO3 · KHF2 · KHSO3 · KHSO4 · KH2AsO4 · KI · KIO3 · KIO4 · KMnO4 · KN3 · KNO2 · KNO3 · KOCN · KOH · KO2 · KPF6 · KSCN · K2CO3 · K2CrO4 · K2Cr2O7 · K2FeO4 · K2MnO4 · K2O · K2O2 · K2PtCl4 · K2PtCl6 · K2S · K2SO3 · K2SO4 · K2SO5 · K2S2O5 · K2S2O7 · K2S2O8 · K2SiO3 · K3[Fe(CN)6] · K3[Fe(C2O4)3] · K4[Fe(CN)6] · K3PO4 · Antiseptics and disinfectants (D08)
Acridine derivatives Ethacridine lactate • 9-Aminoacridine • EuflavineBiguanides and amidines Phenol and derivatives Nitrofuran derivatives NitrofurazoneIodine products Iodine/octylphenoxypolyglycolether • Povidone-iodine# • DiiodohydroxypropaneQuinoline derivatives Quaternary ammonium compounds Mercurial products Silver compounds Alcohols Other Antidotes (V03AB) Nervous system Barbiturate overdoseBemegride • EthamivanBenzodiazepine overdoseGHB overdoseReversal of neuromuscular blockadeCardiovascular Other Paracetamol toxicity (Acetaminophen)OtherPrednisolone/promethazine • oxidizing agent (potassium permanganate) • iodine-131 (Potassium iodide) • Methylthioninium chloride#Emetic Ipecacuanha (Syrup of ipecac) • Copper sulfateCategories:- Potassium compounds
- Permanganates
- Oxidizing agents
- Disinfectants
- Firelighting
- Pyrotechnic oxidizers
- Photographic chemicals
Wikimedia Foundation. 2010.

