- Manganese(II) acetate
-
Manganese(II) acetate[1] 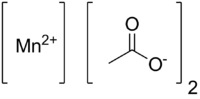 Manganese(II) acetateOther namesManganese diacetate
Manganese(II) acetateOther namesManganese diacetateIdentifiers CAS number 638-38-0, (anhydrous)
[6156-78-1] (tetrahydrate)Jmol-3D images Image 1 - CC([O-])=O.CC([O-])=O.[Mn2+]
Properties Molecular formula Mn(CH3COO)2 (anhydrous)
Mn(CH3COO)2·4H2O (tetrahydrate)Molar mass 173.027 g/mol (anhydrous)
245.087 g/mol (tetrahydrate)Appearance red crystals (anhydrous)
red monoclinic crystals (tetrahydrate)Density ? g/cm3 (anhydrous)
1.59 g/cm3 (tetrahydrate)Melting point 210°C (anhydrous)
80°C (tetrahydrate)Solubility soluble in water, methanol, acetic acid (anhydrous)
soluble in water, ethanol (tetrahydrate)Related compounds Other anions Manganese(II) fluoride
Manganese(II) chloride
Manganese(II) bromideOther cations Zinc acetate
Mercury(II) acetate
Silver acetate acetate (verify) (what is:
acetate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Manganese(II) acetate is the chemical compound with the formula Mn(CH3COO)2. It is used as a desiccant, a catalyst, and as fertilizer.[2]
Reactions
Manganese(II) acetate can be formed by reacting acetic acid with either manganese(II,III) oxide or manganese(II) carbonate[2][3]:
- Mn3O4 + 2CH3COOH → Mn(CH3COO)2 + Mn2O3 + H2O
If manganese(II,III) oxide is used, manganese(III) oxide is produced as a byproduct.
If the anhydrous form needs to be produced, manganese(II) nitrate can be reacted with acetic anhydride.[2]
References
- ^ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 3–354, 4–68, ISBN 0849305942
- ^ a b c Thomas Scott; Mary Eagleson (1994), Concise encyclopedia chemistry, Walter de Gruyter, p. 620, ISBN 3110114518, http://books.google.com/?id=Owuv-c9L_IMC&pg=PA620&dq=%22Manganese%28II%29+acetate%22, retrieved 2009-07-20
- ^ Patnaik, Pradyot (2003), Handbook of Inorganic Chemical Compounds, McGraw-Hill Professional, pp. 81–82, ISBN 0070494398, http://books.google.com/?id=Xqj-TTzkvTEC&pg=PA552&dq=%22Manganese%28II%29+acetate%22, retrieved 2009-07-20
Categories:- Manganese compounds
- Acetates
Wikimedia Foundation. 2010.
