- Manganese(II) nitrate
-
Manganese(II) nitrate 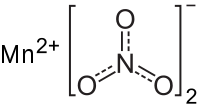 Systematic nameManganese(II) nitrateOther namesManganese dinitrate
Systematic nameManganese(II) nitrateOther namesManganese dinitrateIdentifiers CAS number 10377-66-9  , 20694-39-7 (tetrahydrate)
, 20694-39-7 (tetrahydrate) 
EC number 233-828-8 UN number 2724 Properties Molecular formula Mn(NO3)2 Molar mass 178.95 g/mol Appearance White crystalline solid Density 1.536 g/cm3 Melting point 37 °C, 310 K, 99 °F
Boiling point 100 °C, 373 K, 212 °F
Related compounds Other anions Manganese chloride Other cations Magnesium nitrate
Calcium nitrate nitrate (verify) (what is:
nitrate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Manganese(II) nitrate is an inorganic compound with formula Mn(NO3)2. Each formula unit is composed of one Mn2+ cation and two NO3- anions and varying amounts of water. Most common is the tetrahydrate Mn(NO3)2·4H2O, but mono- and hexahydrates are also known as well as the anhydrous compound. Some of these compounds are useful precursors to the oxides of manganese.[1]
Preparation
Manganese(II) nitrate can be prepared by dissolving manganese(II) carbonate in dilute nitric acid:
- MnCO3 + 2 HNO3 → Mn(NO3)2 + H2O + CO2
It can also be prepared from MnO2 and nitrogen dioxide.[1]
References
- ^ a b Arno H. Reidies, “Manganese Compounds” in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_123
Manganese compounds Categories:- Manganese compounds
- Salts
- Nitrates
- Inorganic compound stubs
Wikimedia Foundation. 2010.

