- Manganese heptoxide
-
Manganese(VII) oxide 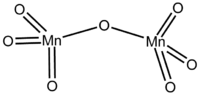
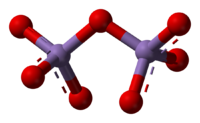
 Manganese(VII) oxideOther namesPermanganic acid
Manganese(VII) oxideOther namesPermanganic acid
Manganic oxide
dimanganese heptoxideIdentifiers CAS number 12057-92-0 Jmol-3D images Image 1 - O=(O=)(O=)MnOMn(=O)(=O)=O
Properties Molecular formula Mn2O7 Molar mass 221.87 g/mol Appearance dark red oil (room temp.), green if in contact with sulfuric acid Density 2.79 g/cm3 Melting point 5.9 °C, 279.1 K
Boiling point explodes on heating
sublimes at −10 °CSolubility in water decomposes to permanganic acid, HMnO4 Structure Crystal structure monoclinic Coordination
geometrybitetrahedral Hazards Main hazards explosive, strong oxidizer, very corrosive Related compounds Related compounds Re2O7
KMnO4
Tc2O7 heptoxide (verify) (what is:
heptoxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Manganese(VII) oxide is an inorganic compound with the formula Mn2O7. This volatile liquid is highly reactive and more often discussed than intentionally prepared. It is a dangerous oxidizer and was first described in 1860.[1]
Properties
The crystalline form of this chemical compound is dark green. It is soluble in carbon tetrachloride, and decomposes when in contact with water. It melts at only 5.9 °C, and sublimes at −10 °C[clarification needed]. These properties indicate a nonpolar molecular species, which is confirmed by its structure. The molecules consist of a pair of tetrahedra that share a common vertex. The vertices are occupied by oxygen atoms and at the centers of the tetrahedra are the Mn(VII) centers. The connectivity is indicated by the formula O3Mn-O-MnO3. The terminal Mn-O distances are 1.585 Å and the bridging oxygen is 1.77 Å distant from the two Mn atoms. The Mn-O-Mn angle is 120.7°.[2]
It contains manganese in its highest oxidation state. This oxidation state, +7, is shared by permanganates, which are more stable compounds.
Pyrosulfate, pyrophosphate, and dichromate adopt structures similar to that of Mn2O7. Probably the most similar main group species is Cl2O7. Focusing on comparisons within the transition metal series, Tc2O7 and Mn2O7 are structurally similar but the Tc-O-Tc angle is 180°. Solid Re2O7 is not molecular but consists of crosslinked Re centers with both tetrahedral and octahedral sites,[3] in the vapour phase it is molecular with a similar structure to Tc2O7.[4]
Synthesis and reactions
Mn2O7 arises as a dark green oil by the addition of concentrated H2SO4 to KMnO4. The reaction initially produces permanganic acid, HMnO4 (structurally, HOMnO3), which is dehydrated by sulfuric acid to form its anhydride, Mn2O7.
- 2 KMnO4 +(cold) 2 H2SO4 → Mn2O7 + H2O + 2 KHSO4
Mn2O7 can react further with sulfuric acid to give the remarkable cation MnO3+, which is isoelectronic with CrO3:[citation needed]
- Mn2O7 + 2 H2SO4 → 2 [MnO3]+[HSO4]− + H2O
Mn2O7 decomposes near room temperature, explosively so at > 55 °C. The explosion can be initiated by striking the sample or by its exposure to oxidizable organic compounds. The products are MnO2 and O2.[5] Ozone is also produced, giving a strong smell to the substance. The ozone can spontaneously ignite a piece of paper impregnated with an alcohol solution. The production of manganese heptoxide is an undesirable byproduct in many situations.[citation needed]
References
- ^ Aschoff, H. Ann. Phys. Chem. Ser. 2 volume 111 (1860) page 217 and page 224.
- ^ Simon, A.; Dronskowski, R.; Krebs, B.; Hettich, B. (1987). "The Crystal Structure of Mn2O7". Angew. Chem. Int. Ed. Engl. 26: 139–140. doi:10.1002/anie.198701391.
- ^ Krebs, B.; Mueller, A.; Beyer, H. H. (1969). "The Crystal Structure of Rhenium(VII) Oxide". Inorganic Chemistry 8: 436–443. doi:10.1021/ic50073a006.
- ^ Wells A.F. (1962) Structural Inorganic Chemistry 3d edition Oxford University Press
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
Manganese compounds Categories:- Explosive chemicals
- Manganese compounds
- Oxides
Wikimedia Foundation. 2010.
