- Sodium permanganate
-
Sodium permanganate 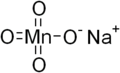
 Sodium manganate(VII)Other namesSodium permanganate, permanganate of sodium
Sodium manganate(VII)Other namesSodium permanganate, permanganate of sodiumIdentifiers CAS number 10101-50-5 
PubChem 24929 ChemSpider 23303 
RTECS number SD6650000 Jmol-3D images Image 1 - [Na+].[O-][Mn](=O)(=O)=O
Properties Molecular formula NaMnO4 Molar mass 141.9254 g/mol
159.94 g/mol (monohydrate)Appearance Red solid Density 1.972 g/cm3 (monohydrate) Melting point 36 °C
Boiling point 100 °C
Solubility in water 900 g/L Hazards Main hazards Oxidizer  permanganate (verify) (what is:
permanganate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium permanganate is the inorganic compound with the formula NaMnO4. It is closely related to the more commonly encountered potassium permanganate, but it is generally less desirable, because it is more expensive, absorbs water from the atmosphere, and has a low melting point. Being about 15 times more soluble than KMnO4, sodium permanganate finds some applications where very high concentrations of MnO4- are sought.
Preparation and properties
Sodium permanganate cannot be prepared analogously to the route to KMnO4 because the required intermediate manganate salt, Na2MnO4, does not form. Thus less direct routes are used including conversion from KMnO4.[1]
Sodium permanganate behaves similarly to potassium permanganate. It dissolves readily in water to give deep purple solutions, evaporation of which gives prismatic purple-black glistening crystals of the monohydrate NaMnO4·H2O. The potassium salt does not form a hydrate. Because of its hygroscopic nature, it is less useful in analytical chemistry than its potassium counterpart.
It can be prepared by the reaction of manganese dioxide with sodium hypochlorite:
- 2 MnO2 + 3 NaClO + 2 NaOH → 2 NaMnO4 + 3 NaCl + H2O
Applications
Because of its high solubility, its aqueous solutions are used as etchants in printed circuitry.[1]
References
- ^ a b Arno H. Reidies "Manganese Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_123
Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Manganese compounds Categories:- Sodium compounds
- Permanganates
- Oxidizing agents
- Disinfectants
- Firelighting
- Inorganic compound stubs
Wikimedia Foundation. 2010.

