- Sodium thiocyanate
-
Sodium thiocyanate 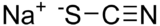

 Sodium thiocyanateOther namesSodium rhodanide
Sodium thiocyanateOther namesSodium rhodanide
Sodium sulfocyanate
Sodium rhodanate
Thiocyanic acid, sodium saltIdentifiers CAS number 540-72-7 
PubChem 516871 ChemSpider 10443 
UNII 5W0K9HKA05 
EC number 208-754-4 ChEBI CHEBI:30952 
RTECS number XL2275000 Jmol-3D images Image 1 - [Na+].[S-]C#N
Properties Molecular formula NaSCN Molar mass 81.072 g/mol Appearance deliquescent colorless crystals Density 1.735 g/cm3 Melting point 287 °C, 560 K, 549 °F
Solubility in water 139 g/100 mL (21 °C) Solubility soluble in acetone, alcohols Refractive index (nD) 1.545 Hazards MSDS ICSC 0675 EU Index 615-004-00-3 EU classification Harmful (Xn) R-phrases R20/21/22, R32, R36, R37, R38 S-phrases S22, S26, S36 NFPA 704 LD50 764 mg/kg (oral, rat)[1] Related compounds Other anions Sodium cyanate
Sodium cyanideOther cations Potassium thiocyanate
Ammonium thiocyanate thiocyanate (verify) (what is:
thiocyanate (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium thiocyanate(sometimes called Sodium sulphocyanide) is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals.[2] Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur:
- 8 NaCN + S8 → 8 NaSCN
Sodium thiocyanate crystallizes in an orthorhombic cell. Each Na+ center is surrounded by three sulfur and three nitrogen ligands provided by the triatomic thiocyanate anion.[3] It is commonly used in the laboratory as a test for the presence of Fe3+ ions.
Applications in chemical synthesis
Sodium thiocyanate is employed to convert alkyl halides into the corresponding alkylthiocyanates. Closely related reagents include ammonium thiocyanate and potassium thiocyanate, which has twice the solubility in water. Silver thiocyanate may be used as well; the precipitation of insoluble silver halides help simplify workup. Treatment of isopropyl bromide with sodium thiocyanate in a hot ethanolic solution affords isopropyl thiocyanate.[4] Protonation of sodium thiocyanate affords isothiocyanic acid, S=C=NH (pKa = -1.28).[5] This species is generated in situ from sodium thiocyanate; it adds to organic amines to afford derivatives of thiourea.[6]
References
- ^ Sodium thiocyanate, chemicalland21.com
- ^ Schwan, A. L. (2001). Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.rs109.
- ^ van Rooyen, P. H.; Boeyens, J. C. A. (1975). "Sodium thiocyanate". Acta Crystallographica B31 (12): 2933–2934. doi:10.1107/S0567740875009326.
- ^ Shriner, R. L. (1943), "Isopropyl Thiocyanate", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv2p0366; Coll. Vol. 2: 366
- ^ Chiang, Y.; Kresge, A. J. (2000). "Determination of the Acidity Constant of Isothiocyanic Acid in Aqueous Solution". Canadian Journal of Chemistry 78 (12): 1627–1628. doi:10.1139/cjc-78-12-1627.
- ^ Allen, C. F. H.; VanAllan, J. (1955), "2-Amino-6-Methylbenzothiazole", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0076; Coll. Vol. 3: 76
Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Categories:- Thiocyanates
- Sodium compounds
Wikimedia Foundation. 2010.

