- Sodium azide
-
Sodium azide 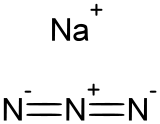

 Other namesSodium trinitride
Other namesSodium trinitride
Smite
AziumIdentifiers CAS number 26628-22-8 
ChemSpider 30958 
EC number 247-852-1 UN number 1687 ChEBI CHEBI:278547 
ChEMBL CHEMBL89295 
RTECS number VY8050000 Jmol-3D images Image 1 - [N-]=[N+]=[N-].[Na+]
Properties Molecular formula NaN3 Molar mass 65.0099 g/mol Appearance white solid Odor odorless Density 1.846 g/cm3 (20 °C) Melting point 275 °C decomp.
Solubility in water 41.7 g/100 mL (17 °C) Solubility in alcohol 0.316 g/100 mL (16 °C) Solubility in ammonia soluble Structure Crystal structure Hexagonal, hR12[1] Space group R-3m, No. 166 Hazards MSDS External MSDS EU Index 011-004-00-7 EU classification Highly toxic (T+)
Dangerous for the environment (N)R-phrases R28, R32, R50/53 S-phrases (S1/2), S28, S45, S60, S61 NFPA 704 Flash point 300 °C LD50 27 mg/kg (oral, rats/mice)[1] Related compounds Other anions Sodium cyanide Other cations Potassium azide
Ammonium azide (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Sodium azide is the inorganic compound with the formula NaN3. This colourless azide salt is the gas-forming component in many car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance and is highly soluble in water. It is extremely toxic.
Contents
Structure
Sodium azide is an ionic solid. Two crystalline forms are known, rhombohedral and hexagonal.[1][2] The azide anion is very similar in each, being centrosymmetric with N–N distances of 1.18 Å. The Na+ ion is pentacoordinated.
Preparation
The common synthesis method is the "Wislicenus process," which proceeds in two steps from ammonia. In the first step, ammonia is converted to sodium amide:
- 2 Na + 2 NH3 → 2 NaNH2 + H2
The sodium amide is subsequently combined with nitrous oxide:
- 2 NaNH2 + N2O → NaN3 + NaOH + NH3
Alternatively the salt can be obtained by the reaction of sodium nitrate with sodium amide.[3]
Applications
Automobile airbags and airplane escape chutes
Older airbag formulations contained mixtures of oxidizers and sodium azide and other agents including igniters and accelerants. An electronic controller detonates this mixture during an automobile crash:
- 2 NaN3 → 2 Na + 3 N2
The same reaction occurs upon heating the salt to approximately 300 °C. The sodium that is formed is a potential hazard itself and, in automobile airbags, it is converted by reaction with other ingredients, such as potassium nitrate and silica. In the latter case, innocuous sodium silicates are generated.[4] Sodium azide is also used in airplane escape chutes. No toxicity has been reported from spent airbags.[5] Newer generation air bags contain nitroguanidine or similar less sensitive explosives.
Organic synthesis
Due to its explosion hazard, sodium azide is of only limited value in industrial scale organic chemistry. In the laboratory, it is used in organic synthesis to introduce the azide functional group by displacement of halides. The azide functional group can thereafter be converted to an amine by reduction with either lithium aluminium hydride or a tertiary phosphine such as triphenylphosphine in the Staudinger reaction, with Raney nickel or with hydrogen sulfide in pyridine.
Inorganic synthesis
Sodium azide is a versatile precursor to other inorganic azide compounds, e.g. lead azide and silver azide, which are used in explosives.
Biochemistry and biomedical uses
Sodium azide is a useful probe reagent, mutagen, and preservative. In hospitals and laboratories, it is a biocide; it is especially important in bulk reagents and stock solutions which may otherwise support bacterial growth where the sodium azide acts as a bacteriostatic by inhibiting cytochrome oxidase in gram-negative bacteria; gram-positive (streptococci, pneumococci, lactobacilli) are resistant,[6] a characteristic similar to antibiotic resistance. It is also used in agriculture for pest control.
Azide inhibits cytochrome oxidase by binding irreversibly to the heme cofactor in a process similar to the action of carbon monoxide. Sodium azide particularly affects organs that undergo high rates of respiration, such as the heart and the brain.
Reactions
Treatment of sodium azide with strong acids gives hydrazoic acid, which is also extremely toxic:
- H+ + N−
3 → HN3
Aqueous solutions contain minute amounts of hydrogen azide, as described by the following equilibrium:
Sodium azide can be destroyed by treatment with nitrous acid solution:[7]
- 2 NaN3 + 2 HNO2 → 3 N2 + 2 NO + 2 NaOH
Safety
Sodium azide is acutely toxic. Symptoms are often compared with those of cyanide. Ingestion of dust or solutions can induce the following symptoms within minutes: rapid breathing, restlessness, dizziness, weakness, headache, nausea and vomiting, rapid heart rate, red eyes (gas or dust exposure), clear drainage from the nose (gas or dust exposure), cough (gas or dust exposure), skin burns and blisters (explosion or direct skin contact). Exposure to a large amount of sodium azide may cause these other health effects as well: convulsions, low blood pressure, low heart rate, loss of consciousness, and lung injury, respiratory failure leading to death.[8]
References
- ^ a b c Stevens E.D., Hope H. (1977). "A Study of the Electron-Density Distribution in Sodium Azide, NaN3". Acta Crystallographica A 33: 723.
- ^ Wells, A. F. (1984), Structural Inorganic Chemistry (5th ed.), Oxford: Clarendon Press, ISBN 0-19-855370-6
- ^ Holleman, A. F.; Wiberg, E. (2001), Inorganic Chemistry, San Diego: Academic Press, ISBN 0-12-352651-5
- ^ Eric A. Betterton (2003). "Environmental Fate of Sodium Azide Derived from Automobile Airbags". Critical Reviews in Environmental Science and Technology 33 (4): 423–458. doi:10.1080/10643380390245002.
- ^ Kent R. Olson (2007). Poisoning and Drug Overdose. McGraw-Hill Professional. ISBN 0071443339. http://books.google.com/?id=25avFCpfQAcC&pg=PA123&lpg=PA123.
- ^ Lichstein, Herman C.; Malcolm H. Soule (1943). "Studies of the Effect of Sodium Azide on Microbic Growth and Respiration: I. The Action of Sodium Azide on Microbic Growth". Journal of Bacteriology 47 (3): 221–230. PMC 373901. PMID 16560767. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=373901.
- ^ Committee on Prudent Practices for Handling, Storage, and Disposal of Chemicals in Laboratories, Board on Chemical Sciences and Technology, Commission on Physical Sciences, Mathematics, and Applications, National Research Council. (1995). Prudent practices in the laboratory: handling and disposal of chemicals. Washington, D.C.: National Academy Press. ISBN 0309052297. http://books.nap.edu/openbook.php?record_id=4911&page=165.
- ^ Mallinckrodt Baker, Inc. (2008-11-21). "MSDS: sodium azide". Environmental Health & Safety, USA. MSDS S2906. http://hazard.com/msds/mf/baker/baker/files/s2906.htm.
External links
- International Chemical Safety Card 0950.
- NIOSH Pocket Guide to Chemical Hazards.
- European Chemicals Bureau.
- Straight Dope on Sodium Azide
Sodium compounds NaAlO2 · NaBH3(CN) · NaBH4 · NaBr · NaBrO3 · NaCH3COO · NaCN · NaC6H5CO2 · NaC6H4(OH)CO2 · NaCl · NaClO · NaClO2 · NaClO3 · NaClO4 · NaF · NaH · NaHCO3 · NaHSO3 · NaHSO4 · NaI · NaIO3 · NaIO4 · NaMnO4 · NaNH2 · NaNO2 · NaNO3 · NaN3 · NaOH · NaO2 · NaPO2H2 · NaReO4 · NaSCN · NaSH · NaTcO4 · NaVO3 · Na2CO3 · Na2C2O4 · Na2CrO4 · Na2Cr2O7 · Na2MnO4 · Na2MoO4 · Na2O · Na2O2 · Na2O(UO3)2 · Na2S · Na2SO3 · Na2SO4 · Na2S2O3 · Na2S2O4 · Na2S2O5 · Na2S2O6 · Na2S2O7 · Na2S2O8 · Na2Se · Na2SeO3 · Na2SeO4 · Na2SiO3 · Na2Te · Na2TeO3 · Na2Ti3O7 · Na2U2O7 · NaWO4 · Na2Zn(OH)4 · Na3N · Na3P · Na3VO4 · Na4Fe(CN)6 · Na5P3O10 · NaBiO3
Categories:- Sodium compounds
- Azides
- Explosive chemicals
Wikimedia Foundation. 2010.

