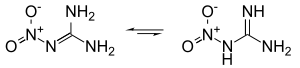- Nitroguanidine
-
Nitroguanidine 
 1-NitroguanidineOther namesPicrite
1-NitroguanidineOther namesPicriteIdentifiers CAS number 556-88-7 
PubChem 11174 ChemSpider 10701 
ChEBI CHEBI:39180 
Jmol-3D images Image 1 - NC(N)=N[N+]([O-])=O
Properties Molecular formula CH4N4O2 Molar mass 104.07 g/mol Appearance Colorless crystalline solid Melting point 232 °C, 505 K, 450 °F
Boiling point 250 °C (decomp.)
Hazards EU Index Not listed Main hazards Explosive Related compounds Related compounds Guanidine
Guanidine nitrate (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Nitroguanidine is a chemical compound. It is a colorless, crystalline solid. It melts at 232 °C and decomposes at 250 °C. It is not flammable and is an extremely low sensitivity explosive; however, its detonation velocity is high.
Contents
Manufacture
Nitroguanidine is manufactured from guanine, a naturally-occurring substance typically found in the excrement of bats and birds (guano). Guanine is extracted from the droppings and is then oxidized to form guanidine.[1] Guanidine is then nitrated to form nitroguanidine. The detailed process used to perform industrial-scale synthesis is considered proprietary and is not available for public release. A laboratory synthesis involves the fusion of sulfamic acid with urea, followed by nitration of the formed guanidine sulfate.[2]
Uses
Explosives
Nitroguanidine is used as an explosive propellant, notably in triple-base smokeless powder. The nitroguanidine reduces the propellant's flash and flame temperature without sacrificing chamber pressure. These are typically used in large bore guns where barrel erosion and flash are particularly important.
Pesticides
Nitroguanidine and its derivatives are used as insecticides, having a comparable effect to nicotine. Derivatives include clothianidin, dinotefuran, imidacloprid, and thiamethoxam.
Biochemistry
The nitrosoylated derivative nitrosoguanidine is often used to mutagenize bacterial cells for biochemical studies.
Structure
Nitroguanidine can exist in distinct tautomeric forms, as a nitroimine (left) or a nitroamine (right). In solution and in the solid state, the nitroimine form predominates.[3][4]
References
- ^ Strecker, A (1861). "Untersuchungen über die chemischen Beziehungen zwischen Guanin, Xanthin, Theobromin, Caffein und Kreatinin". Annalen der Chemie und Pharmacie 118 (2): 151–177. doi:10.1002/jlac.18611180203.
- ^ Axt (August 1, 2007). "Nitroguanidine: from sulphamic acid and urea". Sciencemadness Discussion Board. http://www.sciencemadness.org/talk/viewthread.php?tid=8911.
- ^ Bulusu, S.; Dudley, R. L.; Autera, J. R. (1987). "Structure of nitroguanidine: nitroamine or nitroimine? New NMR evidence from nitrogen-15 labeled sample and nitrogen-15 spin coupling constants". Magnetic Resonance in Chemistry 25 (3): 234–238. doi:10.1002/mrc.1260250311.
- ^ Murmann, R. K.; Glaser, Rainer; Barnes, Charles L. (2005). "Structures of nitroso- and nitroguanidine x - ray crystallography and computational analysis". Journal of Chemical Crystallography 35 (4): 317–325. doi:10.1007/s10870-005-3252-y.
Categories:- Explosive chemicals
- Guanidines
- Nitroamines
- Propellants
Wikimedia Foundation. 2010.