- Nicotine
-
Nicotine 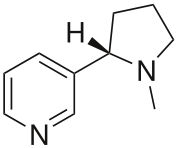
Systematic (IUPAC) name 3-[(2S)-1-methylpyrrolidin-2-yl]pyridine Clinical data Trade names Nicorette, Nicotrol AHFS/Drugs.com monograph Pregnancy cat. D(US) Legal status Unscheduled (AU) ? (UK) ? (US) Dependence liability Medium to high Routes smoked (as smoking tobacco, mapacho, etc.), insufflated (as tobacco snuff or nicotine nasal spray), chewed (as nicotine gum, tobacco gum or chewing tobacco), transdermal (as nicotine patch, nicogel or topical tobacco paste), intrabuccal (as dipping tobacco, snuffs, dissolvable tobacco or creamy snuff), vaporized (as electronic cigarette, etc.), directly inhaled (as nicotine inhaler), oral (as nicotini), buccal (as snus) Pharmacokinetic data Bioavailability 20 to 45% (oral) Metabolism hepatic Half-life 2 hours Identifiers CAS number 54-11-5 
ATC code N07BA01 QP53AX13 PubChem CID 942 DrugBank DB00184 ChemSpider 80863 
UNII 6M3C89ZY6R 
KEGG D03365 
ChEBI CHEBI:17688 
ChEMBL CHEMBL3 
Chemical data Formula C10H14N2 Mol. mass 162.26 g/mol SMILES eMolecules & PubChem Physical data Density 1.01 g/cm³ Melt. point -79 °C (-110 °F) Boiling point 247 °C (477 °F)  (what is this?) (verify)
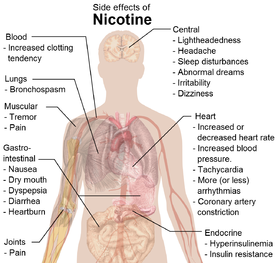
(what is this?) (verify)Nicotine is an alkaloid found in the nightshade family of plants (Solanaceae) that constitutes approximately 0.6–3.0% of the dry weight of tobacco,[1][2] with biosynthesis taking place in the roots and accumulation occurring in the leaves. It functions as an antiherbivore chemical with particular specificity to insects; therefore nicotine was widely used as an insecticide in the past,[3][4] and currently nicotine analogs such as imidacloprid continue to be widely used.
In low concentrations (an average cigarette yields about 1 mg of absorbed nicotine), the substance acts as a stimulant in mammals and is the main factor responsible for the dependence-forming properties of tobacco smoking. According to the American Heart Association, nicotine addiction has historically been one of the hardest addictions to break, while the pharmacological and behavioral characteristics that determine tobacco addiction are similar to those determining addiction to heroin and cocaine. Nicotine content in cigarettes has slowly increased over the years, and one study found that there was an average increase of 1.6% per year between the years of 1998 and 2005. This was found for all major market categories of cigarettes.[5]
Research in 2011 has found that nicotine inhibits chromatin-modifying enzymes (class I and II histone deacetylases) which increases the ability of cocaine to cause an addiction.[6]
History and name
Nicotine is named after the tobacco plant Nicotiana tabacum, which in turn is named after Jean Nicot de Villemain, French ambassador in Portugal, who sent tobacco and seeds from Brazil to Paris in 1560 and promoted their medicinal use. Nicotine was first isolated from the tobacco plant in 1828 by physician Wilhelm Heinrich Posselt and chemist Karl Ludwig Reimann of Germany, who considered it a poison.[7][8] Its chemical empirical formula was described by Melsens in 1843,[9] its structure was discovered by Adolf Pinner and Richard Wolffenstein in 1893, and it was first synthesized by A. Pictet and Crepieux in 1904.[10]
Chemistry
Nicotine is a hygroscopic, oily liquid that is miscible with water in its base form. As a nitrogenous base, nicotine forms salts with acids that are usually solid and water soluble. Nicotine easily penetrates the skin. As shown by the physical data, free base nicotine will burn at a temperature below its boiling point, and its vapors will combust at 308 K (35 °C; 95 °F) in air despite a low vapor pressure. Because of this, most of the nicotine is burned when a cigarette is smoked; however, enough is inhaled to cause pharmacological effects.
Optical activity
Nicotine is optically active, having two enantiomeric forms. The naturally occurring form of nicotine is levorotatory, with [α]D = –166.4°. The dextrorotatory form, (+)-nicotine, has only one-half the physiological activity of (–)-nicotine. [11] The salts of (+)-nicotine are usually dextrorotatory.
Pharmacology
Pharmacokinetics
As nicotine enters the body, it is distributed quickly through the bloodstream and crosses the blood-brain barrier reaching the brain within 10-20 seconds after inhalation.[13] The elimination half-life of nicotine in the body is around two hours.[14]
The amount of nicotine absorbed by the body from smoking depends on many factors, including the types of tobacco, whether the smoke is inhaled, and whether a filter is used. For chewing tobacco, dipping tobacco, snus and snuff, which are held in the mouth between the lip and gum, or taken in the nose, the amount released into the body tends to be much greater than smoked tobacco.[clarification needed][citation needed] Nicotine is metabolized in the liver by cytochrome P450 enzymes (mostly CYP2A6, and also by CYP2B6). A major metabolite is cotinine.
Other primary metabolites include nicotine N'-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide.[15] Under some conditions, other substances may be formed such as myosmine.[16]
Glucuronidation and oxidative metabolism of nicotine to cotinine are both inhibited by menthol, an additive to mentholated cigarettes, thus increasing the half-life of nicotine in vivo.[17]
Detection of use
Medical detection
Nicotine can be quantified in blood, plasma, or urine to confirm a diagnosis of poisoning or to facilitate a medicolegal death investigation. Urinary or salivary cotinine concentrations are frequently measured for the purposes of pre-employment and health insurance medical screening programs. Careful interpretation of results is important, since passive exposure to cigarette smoke can result in significant accumulation of nicotine, followed by the appearance of its metabolites in various body fluids.[18][19] Nicotine use is not regulated in competitive sports programs, yet the drug has been shown to have a significant beneficial effect on athletic performance.[20]
Pharmacodynamics
Nicotine acts on the nicotinic acetylcholine receptors, specifically the ganglion type nicotinic receptor and one CNS nicotinic receptor. The former is present in the adrenal medulla and elsewhere, while the latter is present in the central nervous system (CNS). In small concentrations, nicotine increases the activity of these receptors. Nicotine also has effects on a variety of other neurotransmitters through less direct mechanisms.
In the central nervous system
By binding to nicotinic acetylcholine receptors, nicotine increases the levels of several neurotransmitters - acting as a sort of "volume control". It is thought that increased levels of dopamine in the reward circuits of the brain are responsible for the euphoria and relaxation and eventual addiction caused by nicotine consumption. Nicotine has a higher affinity for acetylcholine receptors in the brain than those in skeletal muscle, though at toxic doses it can induce contractions and respiratory paralysis.[21] Nicotine's selectivity is thought to be due to a particular amino acid difference on these receptor subtypes.[22]
Tobacco smoke contains the monoamine oxidase inhibitors harman, norharman,[23] anabasine, anatabine, and nornicotine. These compounds significantly decrease MAO activity in smokers.[23][24] MAO enzymes break down monoaminergic neurotransmitters such as dopamine, norepinephrine, and serotonin. It is thought that the powerful interaction between the MAOI's and the nicotine is responsible for most of the addictive properties of tobacco smoking. [25] The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats.[26]
Chronic nicotine exposure via tobacco smoking up-regulates alpha4beta2* nAChR in cerebellum and brainstem regions[27][28] but not habenulopeduncular structures.[29] Alpha4beta2 and alpha6beta2 receptors, present in the ventral tegmental area, play a crucial role in mediating the reinforcement effects of nicotine.[30]
In the sympathetic nervous system
Nicotine also activates the sympathetic nervous system,[31] acting via splanchnic nerves to the adrenal medulla, stimulates the release of epinephrine. Acetylcholine released by preganglionic sympathetic fibers of these nerves acts on nicotinic acetylcholine receptors, causing the release of epinephrine (and norepinephrine) into the bloodstream. Nicotine also has an affinity for melanin-containing tissues due to its precursor function in melanin synthesis or due to the irreversible binding of melanin and nicotine. This has been suggested to underlie the increased nicotine dependence and lower smoking cessation rates in darker pigmented individuals.[32]
In adrenal medulla
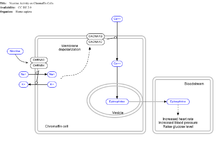
By binding to ganglion type nicotinic receptors in the adrenal medulla nicotine increases flow of adrenaline (epinephrine), a stimulating hormone and neurotransmitter. By binding to the receptors, it causes cell depolarization and an influx of calcium through voltage-gated calcium channels. Calcium triggers the exocytosis of chromaffin granules and thus the release of epinephrine (and norepinephrine) into the bloodstream. The release of epinephrine (adrenaline) causes an increase in heart rate, blood pressure and respiration, as well as higher blood glucose levels.[33]
Nicotine is the natural product of tobacco, having a half-life of 1 to 2 hours. Cotinine is an active metabolite of nicotine that remains in the blood for 18 to 20 hours, making it easier to analyze due to its longer half-life.[34]
Psychoactive effects
Nicotine's mood-altering effects are different by report: in particular it is both a stimulant and a relaxant.[35] First causing a release of glucose from the liver and epinephrine (adrenaline) from the adrenal medulla, it causes stimulation. Users report feelings of relaxation, sharpness, calmness, and alertness.[36] Like any stimulant, it may very rarely cause the often catastrophically uncomfortable neuropsychiatric effect of akathisia. By reducing the appetite and raising the metabolism, some smokers may lose weight as a consequence.[37][38]
When a cigarette is smoked, nicotine-rich blood passes from the lungs to the brain within seven seconds and immediately stimulates the release of many chemical messengers including acetylcholine, norepinephrine, epinephrine, vasopressin, arginine, dopamine, autocrine agents, and beta-endorphin.[39] This release of neurotransmitters and hormones is responsible for most of nicotine's effects. Nicotine appears to enhance concentration[40] and memory due to the increase of acetylcholine.[citation needed] It also appears to enhance alertness due to the increases of acetylcholine and norepinephrine.[citation needed] Arousal is increased by the increase of norepinephrine.[citation needed] Pain is reduced by the increases of acetylcholine and beta-endorphin. Anxiety is reduced by the increase of beta-endorphin. Nicotine also extends the duration of positive effects of dopamine[41] and increases sensitivity in brain reward systems.[42] Most cigarettes (in the smoke inhaled) contain 1 to 3 milligrams of nicotine.[43]
Research suggests that, when smokers wish to achieve a stimulating effect, they take short quick puffs, which produce a low level of blood nicotine.[44] This stimulates nerve transmission. When they wish to relax, they take deep puffs, which produce a high level of blood nicotine, which depresses the passage of nerve impulses, producing a mild sedative effect. At low doses, nicotine potently enhances the actions of norepinephrine and dopamine in the brain, causing a drug effect typical of those of psychostimulants. At higher doses, nicotine enhances the effect of serotonin and opiate activity, producing a calming, pain-killing effect. Nicotine is unique in comparison to most drugs, as its profile changes from stimulant to sedative/pain killer in increasing dosages and use.
Technically, nicotine is not significantly addictive, as nicotine administered alone does not produce significant reinforcing properties.[45] However, after coadministration with an MAOI, such as those found in tobacco, nicotine produces significant behavioral sensitization, a measure of addiction potential. This is similar in effect to amphetamine.[25]
Nicotine gum, usually in 2-mg or 4-mg doses, and nicotine patches are available, as well as smokeless tobacco, nicotine lozenges and electronic cigarettes, which do not have all the other ingredients in tobacco.[46]
A 21 mg patch applied to the left arm. The Cochrane Collaboration finds that NRT increases a quitter's chance of success by 50 to 70%.[47] But in 1990, researchers found that 93% of users returned to smoking within six months.[48]
Dependence and withdrawal
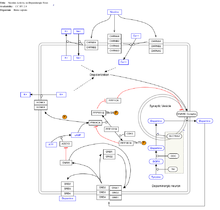
Modern research shows that nicotine acts on the brain to produce a number of effects. Specifically, research examining its addictive nature has been found to show that nicotine activates the Mesolimbic pathway ("reward system") —the circuitry within the brain that regulates feelings of pleasure and euphoria.[49]
Dopamine is one of the key neurotransmitters actively involved in the brain. Research shows that by increasing the levels of dopamine within the reward circuits in the brain, nicotine acts as a chemical with intense addictive qualities. In many studies it has been shown to be more addictive than cocaine and heroin.[50][51][52] Like other physically addictive drugs, nicotine withdrawal causes down-regulation of the production of dopamine and other stimulatory neurotransmitters as the brain attempts to compensate for artificial stimulation. As dopamine regulates the sensitivity of nicotinic acetylcholine receptors decreases. To compensate for this compensatory mechanism, the brain in turn upregulates the number of receptors, convoluting its regulatory effects with compensatory mechanisms meant to counteract other compensatory mechanisms. An example is the increase in norepinephrine, one of the successors to dopamine, which inhibit reuptake of the glutamate receptors,[53] in charge of memory and cognition. The net effect is an increase in reward pathway sensitivity, opposite of other drugs of abuse such as cocaine and heroin, which reduce reward pathway sensitivity.[42] This neuronal brain alteration persists for months after administration ceases.
A study found that nicotine exposure in adolescent mice retards the growth of the dopamine system, thus increasing the risk of substance abuse during adolescence.[54]
Immunology prevention
Because of the severe addictions and the harmful effects of smoking, vaccination protocols have been developed. The principle is under the premise that if an antibody is attached to a nicotine molecule, it will be prevented from diffusing through the capillaries, thus making it less likely that it ever affects the brain by binding to nicotinic acetylcholine receptors.
These include attaching the nicotine molecule to a hapten such as Keyhole limpet hemocyanin or a safe modified bacterial toxin to elicit an active immune response. Often it is added with bovine serum albumin.
Additionally, because of concerns with the unique immune systems of individuals being liable to produce antibodies against endogenous hormones and over the counter drugs, monoclonal antibodies have been developed for short term passive immune protection. They have half-lives varying from hours to weeks. Their half-lives depend on their ability to resist degradation from pinocytosis by epithelial cells.[55][citation needed]
Toxicology
NFPA 704 The LD50 of nicotine is 50 mg/kg for rats and 3 mg/kg for mice. 40–60 mg (0.5-1.0 mg/kg) can be a lethal dosage for adult humans.[56][57] Nicotine therefore has a high toxicity in comparison to many other alkaloids such as cocaine, which has an LD50 of 95.1 mg/kg when administered to mice. It is unlikely that a person would overdose on nicotine through smoking alone, although overdose can occur through combined use of nicotine patches or nicotine gum and cigarettes at the same time.[58] Spilling a high concentration of nicotine onto the skin can cause intoxication or even death, since nicotine readily passes into the bloodstream following dermal contact.[59]
The carcinogenic properties of nicotine in standalone form, separate from tobacco smoke, have not been evaluated by the IARC, and it has not been assigned to an official carcinogen group. The currently available literature indicates that nicotine, on its own, does not actively induce the development of cancer in healthy tissue and has no mutagenic properties.[when?][citation needed] However, nicotine and the increased cholinergic activity it causes have been shown to impede apoptosis, which is one of the methods by which the body destroys unwanted cells (programmed cell death). Since apoptosis helps to remove mutated or damaged cells that may eventually become cancerous, the inhibitory actions of nicotine creates a more favourable environment for cancer to develop.[60] In one study, nicotine administered to mice with tumors caused increases in tumor size (twofold increase), metastasis (nine-fold increase), and tumor recurrence (threefold increase).[61] Through mechanisms like these, nicotine can cause cancer, even if it does not actively induce development of cancer in healthy tissue by being mutagenic.
Though the teratogenic properties of nicotine may or may not yet have been adequately researched, women who use nicotine gum and patches during the early stages of pregnancy face an increased risk of having babies with birth defects, according to a study of around 77,000 pregnant women in Denmark. The study found that women who use nicotine-replacement therapy in the first 12 weeks of pregnancy have a 60 percent greater risk of having babies with birth defects, compared to women who are non-smokers.[citation needed]
Effective April 1, 1990, the Office of Environmental Health Hazard Assessment (OEHHA) of the California Environmental Protection Agency added nicotine to the list of chemicals known to the state to cause developmental toxicity, for the purposes of Proposition 65.[62]
Link to circulatory disease
Nicotine has very powerful effects on arteries throughout the body. Nicotine is a stimulant, it raises blood pressure, and is a vasoconstrictor, making it harder for the heart to pump through the constricted arteries. It causes the body to release its stores of fat and cholesterol into the blood.[63]
It has been speculated[who?] that nicotine increases the risk of blood clots by increasing plasminogen activator inhibitor-1, though this has not been proven. Plasma fibrinogen levels are elevated in smokers and are further elevated during acute COPD exacerbation. Also Factor XIII, which stabilizes fibrin clots, is increased in smokers. But neither of these two effects has been shown to be caused by nicotine [64] as of 2009[update].
Therapeutic uses
The primary therapeutic use of nicotine is in treating nicotine dependence in order to eliminate smoking with the damage it does to health. Controlled levels of nicotine are given to patients through gums, dermal patches, lozenges, electronic/substitute cigarettes or nasal sprays in an effort to wean them off their dependence.
However, in a few situations, smoking has been observed to apparently be of therapeutic value. These are often referred to as "Smoker’s Paradoxes".[65] Although in most cases the actual mechanism is understood only poorly or not at all, it is generally believed that the principal beneficial action is due to the nicotine administered, and that administration of nicotine without smoking may be as beneficial as smoking, without the higher risk to health due to tar and other ingredients found in tobacco.
For instance, recent studies suggest that smokers require less frequent repeated revascularization after percutaneous coronary intervention (PCI).[65] Risk of ulcerative colitis has been frequently shown to be reduced by smokers on a dose-dependent basis; the effect is eliminated if the individual stops smoking.[66][67] Smoking also appears to interfere with development of Kaposi's sarcoma in patients with HIV,[2].[68]
Nicotine reduces the chance of breast cancer among women carrying the very high risk BRCA gene,[69] preeclampsia,[70] and atopic disorders such as allergic asthma.[71] A plausible mechanism of action in these cases may be nicotine acting as an anti-inflammatory agent, and interfering with the inflammation-related disease process, as nicotine has vasoconstrictive effects.[72]
Tobacco smoke has been shown to contain compounds capable of inhibiting MAO. Monoamine oxidase is responsible for the degradation of dopamine in the human brain. When dopamine is broken down by MAO-B, neurotoxic by-products are formed, possibly contributing to Parkinson's and Alzheimers disease.[73] Many such papers regarding Alzheimer's disease[74] and Parkinson's Disease[75] have been published. Recent studies find no beneficial link between smoking and Alzheimer's disease and in some cases, suggest it may actually result in an earlier onset of the disease.[76][77][78][79] However, nicotine has been shown to delay the onset of Parkinson's disease in studies involving monkeys and humans.[80][81][82] However a study has shown a protective effect of nicotine itself on neurons due to nicotine activation of α7-nAChR and the PI3K/Akt pathway which inhibits AIF release and mitochondrial translocation, cytochrome c release and caspase 3 activation. [83]
Recent studies have indicated that nicotine can be used to help adults suffering from autosomal dominant nocturnal frontal lobe epilepsy. The same areas that cause seizures in that form of epilepsy are responsible for processing nicotine in the brain.[84]
Studies suggest a correlation between smoking and schizophrenia, with estimates near 75% for the proportion of schizophrenic patients who smoke. Although the nature of this association remains unclear, it was recently argued that the increased level of smoking in schizophrenia may be due to a desire to self-medicate with nicotine.[85][86] More recent research has found that mildly dependent users got some benefit from nicotine, but not those who were highly dependent.[87] All of these studies are based only on observation, and no interventional (randomized) studies have been done. Research on nicotine as administered through a patch or gum is ongoing.
Nicotine appears to improve ADHD symptoms. Some studies are focusing on benefits of nicotine therapy in adults with ADHD.[88]
Nicotine (in the form of chewing gum or a transdermal patch) is being explored as an experimental treatment for OCD. Small studies show some success, even in otherwise treatment-refractory cases.[89][90][91]
Research as a potential basis for an antipsychotic agent
When the metabolites of nicotine were isolated and their effect on first the animal brain and then the human brain in people with schizophrenia were studied, it was shown that the effects helped with cognitive and negative symptoms of schizophrenia. Therefore, the nicotinergic agents, as antipsychotics which do not contain nicotine but act on the same receptors in the brain are showing promise as adjunct antipsychotics in early stages of FDA studies on schizophrenia. The prepulse inhibition (PPI) is a phenomenon in which a weak prepulse attenuates the response to a subsequent startling stimulus. Therefore, PPI is believed to have face, construct, and predictive validity for the PPI disruption in schizophrenia, and it is widely used as a model to study the neurobiology of this disorder and for screening antipsychotics.[92] Additionally, studies have that there are genes predisposing people with schizophrenia to nicotine use.[93]
Therefore with these factors taken together the heavy usage of cigarettes and other nicotine related products among people with schizophrenia may be explained and novel antipsychotic agents developed that have these effects in a manner that is not harmful and controlled and is a promising arena of research for schizophrenia.
See also
- Nicotiana
- Nicotinic acid (Niacin)
- Drug addiction
- Tobacco cessation
- Chantix
- Zyban
- Nicogel
- Nicotini
- NicVAX
- Nicotine gum
- Nicotine patch
- Nicotine inhaler
- Nicotine nasal spray
- Snus
- Electronic Cigarette
- Psychoactive drug
- Drug Discovery and Development: Nicotinic Acetylcholine Receptor Agonists
- Nicotinic receptor
References
- ^ "Determination of the Nicotine Content of Various Edible Nightshades (Solanaceae) and Their Products and Estimation of the Associated Dietary Nicotine Intake". http://pubs.acs.org/cgi-bin/abstract.cgi/jafcau/1999/47/i08/abs/jf990089w.html. Retrieved 2008-10-05.
- ^ "Smoking and Tobacco Control Monograph No. 9" (PDF). http://dccps.nci.nih.gov/tcrb/monographs/9/m9_3.PDF.
- ^ Rodgman, Alan; Perfetti, Thomas A. (2009) (in English), The chemical components of tobacco and tobacco smoke, Boca Raton, FL: CRC Press, ISBN 1420078836, http://lccn.loc.gov/2008018913
- ^ Some Pesticides Permitted in Organic Gardening
- ^ Connolly, G. N; Alpert, H. R; Wayne, G. F; Koh, H (2007). "Trends in nicotine yield in smoke and its relationship with design characteristics among popular US cigarette brands, 1997-2005". Tobacco Control 16 (5): e5. doi:10.1136/tc.2006.019695. PMID 17897974.
- ^ sciencemag.org - Epigenetics of Nicotine: Another Nail in the Coughing
- ^ W. Posselt and L. Reimann (1828) "Chemische Untersuchung des Tabaks und Darstellung eines eigenthümlich wirksamen Prinzips dieser Pflanze" (Chemical investigation of tobacco and preparation of a characteristically active constituent of this plant), Geiger's Magazin für Pharmacie, volume 6, number 24, pages 138-161.
- ^ Henningfield, JE; Zeller, M (2006). ""Nicotine psychopharmacology", research contributions to United States and global tobacco regulation: A look back and a look forward". Psychopharmacology 184 (3-4): 286–291. doi:10.1007/s00213-006-0308-4. PMID 16463054. http://www.springerlink.com/content/75462q6mq88g4575/fulltext.pdf.
- ^ Melsens (1844). "Über das Nicotin". Journal für Praktische Chemie 32 (1): 372–377. doi:10.1002/prac.18440320155.
- ^ Comptes rendus, 1903, 137, p 860
- ^ Gause, G. F. (1941). Luyet, B. J.. ed. http://www.archive.org/stream/opticalactivityl00gauz/opticalactivityl00gauz_djvu.txt. No. 2 of a series of monographs on general physiology. Normandy, Missouri: Biodynamica. http://www.archive.org/stream/opticalactivityl00gauz/opticalactivityl00gauz_djvu.txt.
- ^ References and comments are found in image description in Commons.
- ^ Le Houezec, J. (Sep 2003). "Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review.". Int J Tuberc Lung Dis 7 (9): 811–9. PMID 12971663.
- ^ Benowitz NL, Jacob P 3rd, Jones RT, Rosenberg J, NL; Jacob P, 3rd; Jones, RT; Rosenberg, J (1982). "Interindividual variability in the metabolism and cardiovascular effects of nicotine in man". J Pharmacol Exp Ther 221 (2): 368–72. PMID 7077531.
- ^ Hukkanen J, Jacob P 3rd, Benowitz NL., J; Jacob P, 3rd; Benowitz, NL (March 2005). "Metabolism and Disposition Kinetics of Nicotine". Pharmacol Rev. 57 (1): 79–115. doi:10.1124/pr.57.1.3. PMID 15734728. http://pharmrev.aspetjournals.org/cgi/content/full/57/1/79.
- ^ http://chromatographyonline.findanalytichem.com/lcgc/News/The-danger-of-third-hand-smoke/ArticleStandard/Article/detail/713385
- ^ Benowitz NL, Herrera B, Jacob P 3rd., NL; Herrera, B; Jacob P, 3rd (2004). "Mentholated Cigarette Smoking Inhibits Nicotine Metabolism". J Pharmacol Exp Ther 310 (3): 1208–15. doi:10.1124/jpet.104.066902. PMID 15084646. http://jpet.aspetjournals.org/cgi/content/abstract/310/3/1208.
- ^ Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 192: 29-60, 2009.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1103-1107.
- ^ Mündel, T. and Jones, D. A. (2006). "Effect of transdermal nicotine administration on exercise endurance in men.". Exp Physiol 91 (4): 705–713. doi:10.1113/expphysiol.2006.033373. PMID 16627574.
- ^ Katzung, Bertram G. Basic & Clinical Pharmacology (Basic and Clinical Pharmacology). New York: McGraw-Hill Medical, 2006. pages 99-105.
- ^ Xiu, Xinan; Puskar, Nyssa L.; Shanata, Jai A. P.; Lester, Henry A.; Dougherty, Dennis A. (2009). "Nicotine binding to brain receptors requires a strong cation– interaction". Nature 458 (7237): 534–537. doi:10.1038/nature07768. PMC 2755585. PMID 19252481. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2755585.
- ^ a b Herraiz T, Chaparro C (2005). "Human monoamine oxidase is inhibited by tobacco smoke: beta-carboline alkaloids act as potent and reversible inhibitors". Biochem. Biophys. Res. Commun. 326 (2): 378–86. doi:10.1016/j.bbrc.2004.11.033. PMID 15582589.
- ^ Fowler JS, Volkow ND, Wang GJ, et al. (1998). "Neuropharmacological actions of cigarette smoke: brain monoamine oxidase B (MAO B) inhibition". J Addict Dis 17 (1): 23–34. doi:10.1300/J069v17n01_03. PMID 9549600.
- ^ a b Villégier AS, Blanc G, Glowinski J, Tassin JP (September 2003). "Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors". Pharmacol. Biochem. Behav. 76 (2): 267–74. doi:10.1016/S0091-3057(03)00223-5. PMID 14592678.
- ^ "Monoamine Oxidase Inhibitors Allow Locomotor and Rewarding Responses to Nicotine". Nature 31 (8). 2006. doi:10.1038/sj.npp.1300987. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=6382912.
- ^ Wüllner U, Gündisch D, Herzog H, et al. (2008). "Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain". Neurosci. Lett. 430 (1): 34–7. doi:10.1016/j.neulet.2007.10.011. PMID 17997038.
- ^ Walsh H, Govind AP, Mastro R, et al. (2008). "Up-regulation of nicotinic receptors by nicotine varies with receptor subtype". J. Biol. Chem. 283 (10): 6022–32. doi:10.1074/jbc.M703432200. PMID 18174175.
- ^ Nguyen HN, Rasmussen BA, Perry DC (2003). "Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography". J. Pharmacol. Exp. Ther. 307 (3): 1090–7. doi:10.1124/jpet.103.056408. PMID 14560040.
- ^ Pons S, Fattore L, Cossu G, et al. (November 2008). "Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration". J. Neurosci. 28 (47): 12318–27. doi:10.1523/JNEUROSCI.3918-08.2008. PMC 2819191. PMID 19020025. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2819191.
- ^ Yoshida T, Sakane N, Umekawa T, Kondo M (Jan 1994). "Effect of nicotine on sympathetic nervous system activity of mice subjected to immobilization stress". Physiol Behav. 55 (1): 53–7. doi:10.1016/0031-9384(94)90009-4. PMID 8140174. http://linkinghub.elsevier.com/retrieve/pii/0031-9384(94)90009-4.
- ^ King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET. Link between facultative melanin and tobacco use among African Americans.(2009). Pharmacol Biochem Behav. 92(4):589-96. doi:10.1016/j.pbb.2009.02.011 PMID 19268687
- ^ Elaine N. Marieb and Katja Hoehn (2007). Human Anatomy & Physiology (7th Ed.). Pearson. pp. ?. ISBN 0-805-35909-5.
- ^ Detection of Cotinine in Blood Plasma by HPLC MS/MS, MIT Undergraduate Research Journal, Volume 8, Spring 2003, Massachusetts Institute of Technology
- ^ Effective Clinical Tobacco Intervention, Therapeutics Letter, issue 21, September–October 1997, University of British Columbia
- ^ Gilbert Lagrue, François Lebargy, Anne Cormier, "From nicotinic receptors to smoking dependence: therapeutic prospects" Alcoologie et Addictologie Vol. : 23, N° : 2S, juin 2001, pages 39S - 42
- ^ Jean-Claude Orsini, "Dependence on tobacco smoking and brain systems controlling glycemia and appetite" Alcoologie et Addictologie Vol. : 23, N° : 2S, juin 2001, pages 28S - 36S
- ^ Smokers lose their appetite : Media Releases : News : The University of Melbourne
- ^ Chemically Correct: Nicotine, Andrew Novick
- ^ Rusted, J; Graupner, O'Connell, Nicholls (1994-05-05). "Does nicotine improve cognitive function?". Psychopharmacology (Springer-Verlag) (115): 547–549. http://www.springerlink.com/content/75034q53031260j8/?p=afde608485604678839ab0e950be77f9&pi=0. Retrieved 2008-11-15.
- ^ http://chronicle.uchicago.edu/020328/nicotine.shtml
- ^ a b Kenny PJ, Markou A (Jun 2006). "Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity". Neuropsychopharmacology 31 (6): 1203–11. doi:10.1038/sj.npp.1300905. PMID 16192981. http://www.nature.com/npp/journal/v31/n6/full/1300905a.html.
- ^ Erowid Nicotine Vault : Dosage
- ^ Einstein, Stanley (1989). Drug and Alcohol Use: Issues and Factors. Springer. pp. 101–118. ISBN 0306413787.
- ^ Guillem K, Vouillac C, Azar MR, et al. (September 2005). "Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats". J. Neurosci. 25 (38): 8593–600. doi:10.1523/JNEUROSCI.2139-05.2005. PMID 16177026.
- ^ E-Cigs Less Dangerous Than Traditional Cigarettes, Researcher Claims
- ^ Stead LF, Perera R, Bullen C, Mant D, Lancaster T. (2008). "Nicotine replacement therapy for smoking cessation". Cochrane Database of Systematic Reviews Art. No.: CD000146. doi:10.1002/14651858.CD000146.pub3. http://www2.cochrane.org/reviews/en/ab000146.html. Retrieved May 22, 2010.
- ^ Millstone, Ken (February 13, 2007). "Nixing the patch: Smokers quit cold turkey". Columbia.edu News Service. http://jscms.jrn.columbia.edu/cns/2007-02-13/millstone-coldturkeyquitters.html. Retrieved May 23, 2010.
- ^ NIDA - Research Report Series - Tobacco Addiction - Extent, Impact, Delivery, and Addictiveness
- ^ Hilts, Philip J. (1994-08-02). "Is Nicotine Addictive? It Depends on Whose Criteria You Use". The New York Times. http://www.nytimes.com/1994/08/02/science/is-nicotine-addictive-it-depends-on-whose-criteria-you-use.html.
- ^ Blakeslee, Sandra (1987-03-29). "NICOTINE: HARDER TO KICK...THAN HEROIN". The New York Times. http://www.nytimes.com/1987/03/29/magazine/nicotine-harder-to-kickthan-heroin.html.
- ^ [1]
- ^ http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WN4-4CCGGN1-9P&_user=10&_coverDate=11%2F30%2F1984&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1520587233&_rerunOrigin=scholar.google&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=5e43884bdf1f204eb2356e02096708bc&searchtype=a
- ^ Nolley EP, Kelley BM (2007). "Adolescent reward system perseveration due to nicotine: studies with methylphenidate". Neurotoxicol Teratol 29 (1): 47–56. doi:10.1016/j.ntt.2006.09.026. PMID 17129706.
- ^ Peterson, Eric C., and Michael Owens. "Designing Immunotherapies to thwart drug abuse." Molecular Interventions June 2009: 119-23. Print.
- ^ Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T (Jul 1994). "Effects of aging on acute toxicity of nicotine in rats". Pharmacol Toxicol. 75 (1): 1–6. doi:10.1111/j.1600-0773.1994.tb00316.x. PMID 7971729.
- ^ IPCS INCHEM
- ^ http://learn.genetics.utah.edu/content/addiction/drugs/overdose.html
- ^ Lockhart LP (1933). "Nicotine poisoning". Br Med J 1: 246–7. doi:10.1136/bmj.1.3762.246-c.
- ^ http://www.plosone.org/article/info:doi/10.1371/journal.pone.0007524
- ^ http://www.plosone.org/article/info:doi/10.1371/journal.pone.0007524
- ^ http://oehha.ca.gov/prop65/prop65_list/files/P65single121809.pdf
- ^ http://www.nature.com/ijo/journal/v25/n8/full/0801654a.html
- ^ http://www.ncbi.nlm.nih.gov/sites/entrez
- ^ a b Cohen, David J.; Michel Doucet, Donald E. Cutlip, Kalon K.L. Ho, Jeffrey J. Popma, Richard E. Kuntz (2001). "Impact of Smoking on Clinical and Angiographic Restenosis After Percutaneous Coronary Intervention". Circulation 104 (7): 773. doi:10.1161/hc3201.094225. PMID 11502701. http://www.data-yard.net/34/circulation_2001_104_773.htm. Retrieved 2006-11-06.
- ^ Longmore, M., Wilkinson, I., Torok, E. Oxford Handbook of Clinical Medicine (Fifth Edition) p. 232
- ^ Green JT, Richardson C, Marshall RW, et al. (Nov 2000). "Nitric oxide mediates a therapeutic effect of nicotine in ulcerative colitis". Aliment Pharmacol Ther. 14 (11): 1429–34. doi:10.1046/j.1365-2036.2000.00847.x. PMID 11069313. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0269-2813&date=2000&volume=14&issue=11&spage=1429.
- ^ cite JNCI J Natl Cancer Inst (2002) 94 (22): 1712-1718. "Smoking Cuts Risk of Rare Cancer". UPI. March 29, 2001. http://www.data-yard.net/10b/kaposi.htm. Retrieved 2006-11-06.
- ^ Recer, Paul (May 19, 1998). "Cigarettes May Have an Up Side". Associated Press. http://www.forces.org/evidence/files/brea.htm. Retrieved 2006-11-06.
- ^ Lain, Kristine Y.; Robert W. Powers, Marijane A. Krohn, Roberta B. Ness, William R. Crombleholme, James M. Roberts (Nov 1991). "Urinary cotinine concentration confirms the reduced risk of preeclampsia with tobacco exposure". American Journal of Obstetrics and Gynecology 181 (5): 908–14. doi:10.1046/j.1365-2222.2001.01096.x. PMID 11422156. http://www.data-yard.net/2/13/ajog.htm. Retrieved 2006-11-06.
- ^ Hjern, A; Hedberg A, Haglund B, Rosen M (June 2001). "Does tobacco smoke prevent atopic disorders? A study of two generations of Swedish residents". Clin Exp Allergy 31 (6): 908–14. doi:10.1046/j.1365-2222.2001.01096.x. PMID 11422156. http://www.data-yard.net/30/asthma.htm. Retrieved 2006-11-06.
- ^ Lisa Melton (June 2006). "Body Blazes". Scientific American: 24. http://www.sciam.com/article.cfm?chanID=sa006&colID=5&articleID=00080902-A2CF-146C-9D1E83414B7F0000.
- ^ Fratiglioni, L; Wang HX (Aug 2000). "Smoking and Parkinson's and Alzheimer's disease: review of the epidemiological studies". Behav Brain Res 113 (1–2): 117–20. doi:10.1016/S0166-4328(00)00206-0. PMID 10942038.
- ^ Thompson, Carol. "Alzheimer's disease is associated with non-smoking". http://www.forces.org/evidence/carol/carol16.htm. Retrieved 2006-11-06.
- ^ Thompson, Carol. "Parkinson's disease is associated with non-smoking". http://www.forces.org/evidence/carol/carol36.htm. Retrieved 2006-11-06.
- ^ "Alzheimer's Starts Earlier for Heavy Drinkers, Smokers". Reuters. 2008-04-17. http://www.reuters.com/article/pressRelease/idUS198346+17-Apr-2008+PRN20080417. Retrieved 2008-06-27.
- ^ Peck, Peggy (2002-07-25). "Smoking Significantly Increases Risk of Alzheimer's Disease Among Those Who Have No Genetic Predisposition". http://www.docguide.com/news/content.nsf/news/8525697700573E1885256C010043BDDC?OpenDocument&c=Smoking%20Related%20Disorders&count=10&id=48dde4a73e09a969852568880078c249. Retrieved 2008-06-27.
- ^ Aggarwal NT, Bienias JL, Bennett DA, et al. (2006). "The relation of cigarette smoking to incident Alzheimer's disease in a biracial urban community population". Neuroepidemiology 26 (3): 140–6. doi:10.1159/000091654. PMID 16493200.
- ^ Lerche Davis,, Jeanie (2004-03-22). "Smoking Speeds Dementia, Alzheimer's Disease". http://www.webmd.com/smoking-cessation/news/20040322/smoking-speeds-dementia-alzheimers-disease. Retrieved 2008-06-27.
- ^ DeNoon, Daniel (2006-08-11). "Nicotine Slows Parkinson's Disease". http://www.webmd.com/parkinsons-disease/news/20060811/nicotine-slows-parkinsons-disease. Retrieved 2009-12-27.
- ^ Peck, Peggy (2002-07-25). "Smoking Significantly Increases Risk of Alzheimer's Disease Among Those Who Have No Genetic Predisposition". http://www.nutraingredients.com/Research/More-vitamin-B6-linked-to-lower-Parkinson-s-risk. Retrieved 2009-12-27.
- ^ Fox, Maggie (2007-10-24). "Nicotine may ease Parkinson's symptoms: U.S. study". Reuters. http://www.reuters.com/article/idUSN2431402020071024. Retrieved 2009-12-27.
- ^ J Neurochem. 2011 Sep 2. doi: 10.1111/j.1471-4159.2011.07466.x.
- ^ "Nicotine as an antiepileptic agent in ADNFLE: An n-of-one study". http://www.cnsforum.com/commenteditem/3c5dccdc-27fb-4b80-9516-ab81e3e4ea6c/default.aspx.
- ^ de Leon J, Tracy J, McCann E, McGrory A, Diaz FJ (Jul 2002). "Schizophrenia and tobacco smoking: a replication study in another US psychiatric hospital". Schizophr Res. 56 (1-2): 55–65. doi:10.1016/S0920-9964(01)00192-X. PMID 12084420. http://linkinghub.elsevier.com/retrieve/pii/S092099640100192X.
- ^ de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (Mar 1995). "Schizophrenia and smoking: an epidemiological survey in a state hospital". Am J Psychiatry 152 (3): 453–5. PMID 7864277. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=7864277.
- ^ Aguilar MC, Gurpegui M, Diaz FJ, de Leon J (Mar 2005). "Nicotine dependence and symptoms in schizophrenia: naturalistic study of complex interactions". Br J Psychiatry 186: 215–21. doi:10.1192/bjp.186.3.215. PMID 15738502.
- ^ "Attention-Deficit Hyperactivity Disorder". http://adam.about.com/reports/000030_1.htm. Retrieved 21 September 2009.
- ^ Pasquini M, Garavini A, Biondi M (January 2005). "Nicotine augmentation for refractory obsessive-compulsive disorder. A case report". Prog. Neuropsychopharmacol. Biol. Psychiatry 29 (1): 157–9. doi:10.1016/j.pnpbp.2004.08.011. PMID 15610960.
- ^ Lundberg S, Carlsson A, Norfeldt P, Carlsson ML (November 2004). "Nicotine treatment of obsessive-compulsive disorder". Prog. Neuropsychopharmacol. Biol. Psychiatry 28 (7): 1195–9. doi:10.1016/j.pnpbp.2004.06.014. PMID 15610934.
- ^ Tizabi Y, Louis VA, Taylor CT, Waxman D, Culver KE, Szechtman H (January 2002). "Effect of nicotine on quinpirole-induced checking behavior in rats: implications for obsessive-compulsive disorder". Biol. Psychiatry 51 (2): 164–71. doi:10.1016/S0006-3223(01)01207-0. PMID 11822995. http://linkinghub.elsevier.com/retrieve/pii/S0006322301012070.
- ^ Suemaru K, Kohnomi S, Umeda K, Araki H., K; Kohnomi, S; Umeda, K; Araki, H (2008). "Alpha7 nicotinic receptor agonists have reported to reverse the PPI disruption" (in Japanese). Nihon Shinkei Seishin Yakurigaku Zasshi 28 (3): 121–6. PMID 18646597.
- ^ De Luca V, Wong AH, Muller DJ, Wong GW, Tyndale RF, Kennedy JL. (2004). "Evidence of association between smoking and alpha7 nicotinic receptor subunit gene in schizophrenia patients". Neuropsychopharmacology 29 (8): 1522–6. doi:10.1038/sj.npp.1300466. PMID 15100704.
Further reading
- Bilkei-Gorzo A, Rácz I, Michel K, Darvas M, Rafael Maldonado López, Zimmer A. (2008). "A common genetic predisposition to stress sensitivity and stress-induced nicotine craving". Biol. Psychiatry 63 (2): 164–71. doi:10.1016/j.biopsych.2007.02.010. PMID 17570348.
- Willoughby JO, Pope KJ, Eaton V (Sep 2003). "Nicotine as an antiepileptic agent in ADNFLE: an N-of-one study". Epilepsia 44 (9): 1238–40. doi:10.1046/j.1528-1157.2003.11903.x. PMID 12919397. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0013-9580&date=2003&volume=44&issue=9&spage=1238.
- Minna JD (Jan 2003). "Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer". J Clin Invest. 111 (1): 31–3. doi:10.1172/JCI17492. PMC 151841. PMID 12511585. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=151841.
- Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG (Mar 2005). "Gender: a major determinant of brain response to nicotine". Int J Neuropsychopharmacol. 8 (1): 17–26. doi:10.1017/S1461145704004730. PMID 15579215. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=282494.
- West KA, Brognard J, Clark AS, et al. (Jan 2003). "Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells". J Clin Invest. 111 (1): 81–90. doi:10.1172/JCI16147. PMC 151834. PMID 12511591. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=151834.
- National Institute on Drug Abuse
- Erowid information on tobacco
External links
Addiction Drugs Food Behavioral Other addictions Stimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic aminesAntiaddictives (N07B) Nicotine dependence/
(Nicotinic agonist)Nicotine • Dianicline • Varenicline • Lobeline • Mecamylamine • Scopolamine
NDRI (Bupropion) • AA (Clonidine) • CB1 (Surinabant)Alcohol dependence Opioid dependence Stimulant dependence Benzodiazepine dependence Cocaine dependence Sedative-Hypnotic dependence Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • Pralidoxime
Wikimedia Foundation. 2010.
Look at other dictionaries:
NICOTINE — Lorsqu’en 1492 Christophe Colomb et ses marins découvrent l’Amérique, ils trouvent dans l’île de San Salvador des hommes qui portent à leur bouche une certaine herbe qu’ils allument avec un tison pour se régaler de son parfum; ce sont sans doute… … Encyclopédie Universelle
Nicotine — Structure de la nicotine Général Nom IUPAC (S) 3 (1 méthyl 2 pyrrolidinyl)pyridine … Wikipédia en Français
Nicotine — Desarrollador Hyriand Página web oficial Información general Última versión estable 1.0.8 13 de febrero de … Wikipedia Español
Nicotine — Nic o*tine (? or ?), n. [F. nicotine. See {Nicotian}.] (Chem.) An alkaloid which is the active principle of tobacco ({C10H14N2}). It occurs in tobacco plants ({Nicotiana tabacum} and {Nicotiana rusticum}) to the extent of 2 to 8%, in combination… … The Collaborative International Dictionary of English
nicotine — (n.) poisonous alkaloid found in tobacco leaves, 1819, from Fr. nicotine, earlier nicotiane, from Mod.L. Nicotiana, formal botanical name for the tobacco plant, named for Jean Nicot (c.1530 1600), French ambassador to Portugal, who sent tobacco… … Etymology dictionary
nicotine — nicotine. См. никотин. (Источник: «Англо русский толковый словарь генетических терминов». Арефьев В.А., Лисовенко Л.А., Москва: Изд во ВНИРО, 1995 г.) … Молекулярная биология и генетика. Толковый словарь.
nicotine — ► NOUN ▪ a toxic oily liquid which is the chief active constituent of tobacco. ORIGIN named after Jaques Nicot, a 16th century diplomat who introduced tobacco to France … English terms dictionary
nicotine — [nik′ə tēn΄, nik΄ə tēn′] n. [Fr < nicotiane, the tobacco plant < ModL nicotiana (herba), Nicot s (plant), after Jean Nicot (1530 1600), Fr ambassador at Lisbon, who first introduced tobacco into France (1560)] a toxic, addictive, water… … English World dictionary
nicotine — nicotined, adj. nicotineless, adj. /nik euh teen , tin, nik euh teen /, n. Chem. a colorless, oily, water soluble, highly toxic, liquid alkaloid, C10H14N2, found in tobacco and valued as an insecticide. [1810 20; < F; see NICOTIANA, INE2] * * *… … Universalium
nicotine — 1 Methyl 2 (3 pyridyl)pyrrolidine; a poisonous volatile alkaloid derived from tobacco (Nicotiana spp.) and responsible for many of the effects of tobacco; it first stimulates (small doses), then depresses (large doses) at autonomic ganglia and… … Medical dictionary