- Baclofen
-
Baclofen 
Systematic (IUPAC) name (RS)-4-amino-3-(4-chlorophenyl)butanoic acid Clinical data Trade names Lioresal AHFS/Drugs.com monograph Licence data US FDA:link Pregnancy cat. C(US) Legal status ℞-only (US) Routes Oral, intrathecal Pharmacokinetic data Bioavailability well absorbed Protein binding 30% Metabolism 85% excreted in urine/faeces unchanged. 15% metabolised by deamination Half-life 1.5 to 4 hours Excretion renal (70-80%) Identifiers CAS number 1134-47-0 
ATC code M03BX01 PubChem CID 2284 IUPHAR ligand 1084 DrugBank APRD00551 ChemSpider 2197 
UNII H789N3FKE8 
KEGG D00241 
ChEBI CHEBI:2972 
ChEMBL CHEMBL701 
Chemical data Formula C10H12ClNO2 Mol. mass 213.661 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Baclofen (brand names Kemstro, Lioresal, and Gablofen) is a derivative of gamma-aminobutyric acid (GABA). It is primarily used to treat spasticity and is under investigation for the treatment of alcoholism.
It is an agonist for the GABAB receptors.[1][2] Its beneficial effects in spasticity result from actions at spinal and supraspinal sites. Baclofen can also be used to treat hiccups, and has been shown to prevent rises in body temperature induced by the drug MDMA in rats.[3]
In addition, research has shown baclofen to be effective in the treatment of alcohol dependence and withdrawal, by inhibiting both withdrawal symptoms and cravings.[4][5]
A very beneficial property of baclofen is that tolerance does not seem to occur to any significant degree — baclofen retains its therapeutic anti-spasmodic effects even after many years of continued use.[6] However, oral dosage must be carefully regulated; significantly high doses of the drug, particularly 80 milligrams per day or higher, can cause excessive drowsiness that can interfere with daily function.[citation needed]
Contents
Use
Baclofen is widely used for the treatment of spastic movement disorders, especially in instances of spinal cord injury, spastic diplegia cerebral palsy, multiple sclerosis, amyotrophic lateral sclerosis (Lou Gehrig's disease), peripheral neuropathy and trigeminal and glossopharyngeal neuralgias.[citation needed]
Baclofen is often the primary, or at least preliminary, drug treatment for spastic diplegia and more general spasticity-based mobility impairments. Research has not, however, shown it to be consistently effective in improving function for people with these issues.[7][8] Sometimes, effects are immediate and clear; other times, effects may be mild, vague, or nonexistent. What is known for sure is that, for unknown reasons, in about 5% of the spastic cerebral palsy people who try intrathecally-administered baclofen, the drug has no effect whatsoever on the person's spasticity. It does appear that intrathecal baclofen may be more effective than oral baclofen, but some believe the long-term risks of an intrathecal pump (including potential sudden infection, sudden malfunction leading to coma and death, etc.) outweigh the potential benefits. Many clinicians and/or patients choose not to administer baclofen intrathecally at all for such reasons. (Most manufacturers of intrathecal pumps strongly disagree with the belief that an intrathecal pump carries with it a significant or even remote risk of serious complication, despite such things having occurred often enough to warrant concern.) Manufacturers argue that a pump that fails shuts down, but this does not itself mean that a suddenly punctured or similarly compromised pump would not pose a deathly risk to its wearer.
Intrathecal pumps are much more commonly used for the delivery of morphine than baclofen alone, although a mixture of both for chronic pain sufferers is common. A failure of the type alleged with an intrathecal pump with morphine would be fatal if it occurred when the pump was full or near to full where even a small amount suddenly was released into the Cerebral Spinal Fluid. It would likely be fatal if released into the abdominal cavity. Similar circumstances are used as arguments against intrathecal baclofen.
Baclofen has been shown to be as effective as diazepam in uncomplicated alcohol withdrawal syndrome.[4] An Italian study showed that it was effective in promoting alcohol abstinence in patients with severe liver cirrhosis.[9]
Due to its phenethylamine structure and properties, baclofen is useful in the treatment of tardive dyskinesia.[10]
Oral baclofen, taken as needed, can be used to alleviate painful low back spasms in patients with spinal abnormalities. It is generally seen, for this use, as more effective than medications such as cyclobenzaprine e.g. Flexeril and Metaxalone e.g. Skelaxin but not as strong (or abusable) as carisoprodol e.g. Soma.
Mechanism of action
Baclofen produces its effects by activating the GABAB receptor, similar to the drug GHB which also activates this receptor and shares some of its effects. However, baclofen does not have significant affinity for the GHB receptor, and has no known abuse potential.[11][12] The modulation of the GABAB receptor is what produces baclofen's range of therapeutic properties.
History
Historically baclofen was designed to be a drug for epilepsy. It was synthesized for the first time in Ciba-Geigy by the Swiss chemist Heinrich Keberle in 1962.[13] The effect on epilepsy was disappointing but it was found that in certain patients spasticity decreased. Baclofen was and is still given orally with variable effects. In severely affected children, the oral dose is so high that side effects appear and the treatment loses its benefit. How and when baclofen came to be used in the spinal sac is not really clear but this is now an established method for the treatment of spasticity in many conditions.
As a treatment for alcohol and other addictions
Dr. Olivier Ameisen, a French-American associate professor of medicine and a cardiologist at Weill Cornell Medical College of Cornell University, reported in 2004 that he successfully used baclofen to completely suppress his own alcohol addiction.[14] Ameisen called for randomized trials of high-dose baclofen to be conducted to test the therapeutic model he had proposed.[15] Ameisen believes, based on his own experience and other anecdotal evidence, that baclofen acts on some mechanism within the brains of addicts to suppress cravings brought on by addiction to various substances such as alcohol, cocaine, and heroin.[15] However, a few randomized clinical trials have shown mixed results to date.[15] A more extensive clinical trial will take place starting in 2011.[15]
Ameisen authored Le Dernier Verre (The Last Glass, titled The End of My Addiction and Heal Thyself in English) to inform public opinion and physicians.[16][17]
Based on Ameisen's therapeutic model, some trials have been conducted in using baclofen to treat cocaine addiction. In 2007, an Italian team demonstrated the effectiveness and the safety of baclofen as a treatment for alcohol addiction[5] There is also a report that baclofen has beneficial role in the management of reflux disease.[18]
Inspired by reading Olivier Ameisen’s The End of My Addiction, an anonymous donor gave $750,000 to the University of Amsterdam (UvA) in the Netherlands to initiate the clinical trial of high-dose baclofen Ameisen had called for since 2004.[15] The trial is scheduled to start in January 2011 and will be led by the team of Pr. Dr. Reinout Wiers. Ameisen has been contacted by the team.[19] In May 2011 a Scottish team from Glasgow presented: "Baclofen at a Tailored Dose Reduces Alcohol Use, Craving and Consequences of Drinking in Alcoholics with Medical Disease due to Alcohol Dependence" at the "Royal College of Pscychiatrists Faculty of Addictions Psychiatry Annual Meeting". They used doses between 15 and 360 mg of baclofen per day and winning the conference prize for the best poster raised the profile and increased interest in baclofen as anticraving drug.
In 2010, a small study in Minnesota replicated findings from Italy indicating that off-label use of the gamma-aminobutyric acid–derivative baclofen is effective in treating symptoms of alcohol withdrawal syndrome (AWS).[20]
Description of compound
Baclofen is a white (or off white) mostly odorless crystalline powder, with a molecular weight of 213.66 g/mol. It is slightly soluble in water, very slightly soluble in methanol, and insoluble in chloroform.
Pharmacokinetics
The drug is rapidly absorbed after oral administration and is widely distributed throughout the body. Biotransformation is low and the drug is predominantly excreted in the unchanged form by the kidneys.
Routes of administration
Baclofen can be administered either orally or intrathecally (directly into the cerebral spinal fluid) using a pump implanted under the skin.
Oral doses are typically 5–20 mg given 2-4 times/day. The maximum daily dosage is 80 mg. Titration generally occurs in 3 day increments e.g. 20 mg/day for days 1-3, 40 mg/day for days 4-6, 60 mg/day for days 7-9, and finally 80 mg/day for days 10-12. Careful titration of the medication upwards from the minimum dose should provide a good enough indicator of how much is needed for adequate spasticity relief. Baclofen can also be used on an as needed basis, such as for pain relief of low back spasms; in these cases dosing is the same (5–20 mg given 2-4 times/day, with the same 80 mg/day maximum) but taken only when symptoms arise.
Intrathecal pumps offer much lower doses of baclofen because they are designed to deliver the medication directly to the spinal fluid rather than going through the digestive and blood system first. They are often preferred in spasticity patients such as those with spastic diplegia, as very little of the oral dose actually reaches the spinal fluid. Besides those with spasticity, intrathecal administration is also used in patients with multiple sclerosis who have severe painful spasms which are not controllable by oral baclofen. With pump administration, a test dose is first injected into the spinal fluid to assess the effect, and if successful in relieving spasticity, a chronic intrathecal catheter is inserted from the spine through to the abdomen and attached to the pump which is implanted under the abdomen's skin, usually by the ribcage. The pump is computer-controlled for automatic dosage and the reservoir in the pump can be replenished by percutaneous injection.
In about 5% of patients, the intrathecal route has absolutely no effect on the nervous system, no matter how great a dose is administered.[citation needed] A similar lack of any effect have been reported by those with spasticity who try the oral route,[citation needed] but for some, the oral route works while the intrathcal route does not. Again, there are no known clinical theories as to why these discrepancies are present in the baclofen-spastic CP pairing. Additionally, for some people with spasticity, a lower dose of baclofen may be less effective, while for others that same dose will be very effective. This is why clinicians always insist to a spastic diplegic or similar person that s/he must start out with a low dose of baclofen and increase the dosage slowly.
Dosage
Baclofen therapy is usually started with an initial low dose of about 10 mg daily in divided doses and gradually titrated up in a stepwise fashion until symptomatic relief occurs. The usual maximum dose is 80 mg per day. However, it is common to titrate the dosage until symptomatic control or relief is attained; there is no inherent danger of baclofen in any dose in people with non-impaired renal function as long as the dose is titrated properly, so as not to cause undue side-effects such as oversedation. It is more common to use higher dosages when attempting to treat drug cravings instead of spasticity.[5][21]
Withdrawal syndrome
Discontinuation of baclofen can be associated with a withdrawal syndrome which resembles benzodiazepine withdrawal and alcohol withdrawal. Withdrawal symptoms are more likely if baclofen is used for long periods of time (more than a couple of months) and can occur from low or high doses. The severity of baclofen withdrawal depends on the rate at which baclofen is discontinued. Thus to minimise baclofen withdrawal symptoms the dose should be tapered down slowly when discontinuing baclofen therapy. Abrupt withdrawal is most likely to result in severe withdrawal symptoms. Acute withdrawal symptoms can be stopped by recommencing baclofen.[22]
Withdrawal symptoms may include auditory hallucinations, visual hallucinations, tactile hallucinations, delusions, confusion, agitation, delirium, disorientation, fluctuation of consciousness, insomnia, dizziness, Nausea, Feeling Faint, inattention, memory impairments, perceptual disturbances, pruritus/itching, anxiety, depersonalization, hypertonia, hyperthermia, formal thought disorder, psychosis, mania, mood disturbances, restlessness, and behavioral disturbances, tachycardia, seizures, tremors, autonomic dysfunction, hyperpyrexia, extreme muscle rigidity resembling neuroleptic malignant syndrome and rebound spasticity.[22][23]
Overdose
Symptoms of a baclofen overdose include vomiting, weakness, drowsiness, slow breathing, seizures, unusual pupil size, pruritus/itching and coma.
Chemistry
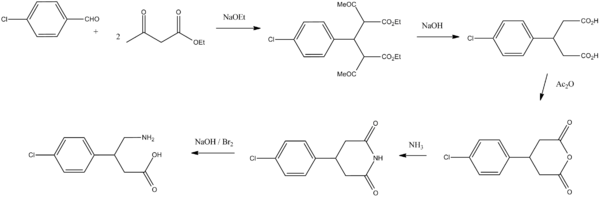
Baclofen, 4-amino-3-(4-chlorophenyl)butyric acid, is synthesized in two ways. According to the first, 4-chlorobenzaldehyde is condensed with two moles of acetoacetic ester, giving the product, which initially undergoes alkaline hydrolysis and decarboxylation forming 3-(4-chlorphenyl)glutaric acid. Dehydration of this gives 3-(4-chlorophenyl)glutaric acid anhydride, and further treatment with ammonia gives the corresponding glutarimide. Reacting this with an alkaline solution of a halogen (Hofmann rearrangement) gives baclofen.[24][25]
The second way of synthesizing baclofen is started from ethyl ester of 4-chlorocinnamic acid. Adding nitromethane to this in the presence of base gives ethyl ester of β-(4-chlorophenyl)-γ-nitrobutyric acid, the nitro group of which is reduced by hydrogen over Raney nickel to the ethyl ester of β-(4-chlorophenyl)-γ-aminobutyric acid, which is further hydrolyzed into the desired baclofen.[26]
References
- ^ Mezler M, Müller T, Raming K (February 2001). "Cloning and functional expression of GABA(B) receptors from Drosophila". Eur. J. Neurosci. 13 (3): 477–86. doi:10.1046/j.1460-9568.2001.01410.x. PMID 11168554.
- ^ Dzitoyeva S, Dimitrijevic N, Manev H (April 2003). "Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence". Proc. Natl. Acad. Sci. U.S.A. 100 (9): 5485–90. Bibcode 2003PNAS..100.5485D. doi:10.1073/pnas.0830111100. PMC 154371. PMID 12692303. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=154371. Retrieved 2009-03-26.
- ^ Bexis S, Phillis BD, Ong J, White JM, Irvine RJ. (2004-04-09). "Baclofen prevents MDMA-induced rise in core body temperature in rats". Drug and Alcohol Dependence 74 (1): 89–96. doi:10.1016/j.drugalcdep.2003.12.004. PMID 15072812.
- ^ a b Addolorato G; Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G (March 2006). "Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam". Am J Med 119 (3): 276.e13–8. doi:10.1016/j.amjmed.2005.08.042. PMID 16490478.
- ^ a b c Addolorato, G.; Leggio, L.; Ferrulli, A.; Cardone, S.; Vonghia, L.; Mirijello, A.; Abenavoli, L.; D'Angelo, C. et al. (Dec 2007). "Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study". Lancet 370 (9603): 1915–22. doi:10.1016/S0140-6736(07)61814-5. PMID 18068515.
- ^ Gaillard JM (May-Jun 1977). "Comparison of two muscle relaxant drugs on human sleep: diazepam and parachlorophenylgaba". Acta Psychiatr Belg 77 (3): 410–25. PMID 200069.
- ^ Taricco, Mariangela; Adone, Roberto; Pagliacci, Christina; Telaro, Elena (2000). "Pharmacological interventions for spasticity following spinal cord injury". In Taricco, Mariangela. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD001131.
- ^ Shakespeare, David; Boggild, Mike; Young, Carolyn A (2003). "Anti-spasticity agents for multiple sclerosis". In Shakespeare, David. Cochrane Database of Systematic Reviews. doi:10.1002/14651858.CD001332.
- ^ Susan Jeffrey (December 7, 2007). Baclofen Aids in Alcohol Abstinence in Cirrhosis Patients. Medscape. http://www.medscape.com/viewarticle/567102
- ^ Wolf ME; Keener S, Mathis P, Mosnaim AD (1983). "Phenethylamine-like properties of baclofen". Neuropsychobiology 9 (4): 219–22. doi:10.1159/000117968. PMID 6646393.
- ^ Carter, LP.; Koek, W.; France, CP. (Jan 2009). "Behavioral analyses of GHB: receptor mechanisms" (PDF). Pharmacol Ther 121 (1): 100–14. doi:10.1016/j.pharmthera.2008.10.003. PMC 2631377. PMID 19010351. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2631377.
- ^ Carter LP, Koek W, France CP (October 2008). "Behavioral analyses of GHB: Receptor mechanisms". Pharmacol. Ther. 121 (1): 100–14. doi:10.1016/j.pharmthera.2008.10.003. PMC 2631377. PMID 19010351. http://linkinghub.elsevier.com/retrieve/pii/S0163-7258(08)00196-4.
- ^ Froestl, Wolfgang; Ragavendran, JV; Sriram, D (2010). [http:/www.bentham.org/rpcn/samples/rpcn1-1/Yogeeswari.pdf Chemistry and Pharmacology of GABAB Receptor Ligands]. "GABAReceptor Pharmacology - A Tribute to Norman Bowery". Recent patents on CNS drug discovery. Advances in Pharmacology 58 (1): 19–62. doi:10.1016/S1054-3589(10)58002-5. ISBN 9780123786470. PMID 18221197. http:/www.bentham.org/rpcn/samples/rpcn1-1/Yogeeswari.pdf.
- ^ Olivier Ameisen (2005 Mar-Apr 2005). "Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician". Alcohol and Alcoholism 40 (2): 147–150. doi:10.1093/alcalc/agh130. PMID 15596425. http://alcalc.oxfordjournals.org/cgi/content/abstract/40/2/147.
- ^ a b c d e Martin Enserink (6 May 2011). "Anonymous Alcoholic Bankrolls Trial of Controversial Therapy". Science 332 (6030): 653. doi:10.1126/science.332.6030.653. PMID 21551041. http://www.sciencemag.org/content/332/6030/653.full. Retrieved 2011-05-31.
- ^ "Cheap pill is 'miracle cure' for alcoholism". Independent.ie. 2008-12-08. http://www.independent.ie/health/latest-news/cheap-pill-is-miracle-cure-for-alcoholism-1566925.html.
- ^ "France abuzz over alcoholic 'cure'". BBC News. 2008-12-06. http://news.bbc.co.uk/2/hi/europe/7768141.stm. Retrieved December 6, 2008.
- ^ Zhang Q et al. (2001). "Control of transient lower oesophageal sphincter relaxations and reflux by the GABAb agonist baclofen in patients with gastro-oesophageal reflux disease". Gut 50 (1): 19–24. doi:10.1136/gut.50.1.19. PMC 1773078. PMID 11772961. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1773078.
- ^ Olivier Ameisen, MD
- ^ Medscape: Medscape Access
- ^ "Kemstro (Baclofen) drug indications and dosage - prescription drugs and medications". RxList. pp. 2. http://www.rxlist.com/cgi/generic/baclofen_ids.htm. Retrieved September 21, 2008.
- ^ a b Leo RJ; Baer D (Nov-Dec 2005). "Delirium Associated With Baclofen Withdrawal: A Review of Common Presentations and Management Strategies". Psychosomatics 46 (6): 503–507. doi:10.1176/appi.psy.46.6.503. PMID 16288128. http://psy.psychiatryonline.org/cgi/content/full/46/6/503.
- ^ Grenier B, Mesli A; Cales J, Castel JP, Maurette P (1996). "[Severe hyperthermia caused by sudden withdrawal of continuous intrathecal administration of baclofen]". Ann Fr Anesth Reanim 15 (5): 659–62. PMID 9033759.
- ^ W. Max, F.J. Werner, U.S. Patent 3,471,548 (1969)
- ^ W. Max, F.J. Werner, U.S. Patent 3,634,428 (1972)
- ^ F. Uchimaru, M. Sato, E. Kasasayama, M. Shiamuzu, H. Takashi, JP 45016692 (1970)
External links
- Intrathecal Baclofen Therapy Cleveland Clinic Information Center. 15 June 2001.
- FYI: Baclofen (Lioresal), from the New York State Office of Alcoholism and Substance Abuse Services
- Intrathecal Baclofen, from University Hospital Nottingham, Queen's Medical Centre)
- U.S. National Library of Medicine: Drug Information Portal - Baclofen
Skeletal muscle relaxants (M03) Peripherally acting
(primarily antinicotinic,
NMJ block)Curare alkaloidsultra-short duration: Gantacurium
short duration: Mivacurium • Chandonium
intermediate duration: Atracurium • Cisatracurium • Fazadinium • Rocuronium • Vecuronium
long duration: Doxacurium • Dimethyltubocurarine • Pancuronium • Pipecuronium • Laudexium • Gallamine
unsorted: Hexafluronium (Hexafluorenium)Choline derivatives: Suxamethonium (Succinylcholine)
Polyalkylene derivatives: HexamethoniumCentrally acting Carbamic acid estersBenzodiazepinesAnticholinergics (Antimuscarinics)OtherBaclofen • Chlormezanone • Chlorphenesin • Chlorzoxazone • Donepezil • Eperisone • Flopropione • Mephenesin • Mephenoxalone • Metaxalone • Phenyramidol • Pridinol • Promoxolane • Quinine • Thiocolchicoside • Tizanidine • Tolperisone • TrazodoneDirectly acting Antiaddictives (N07B) Nicotine dependence/
(Nicotinic agonist)Nicotine • Dianicline • Varenicline • Lobeline • Mecamylamine • Scopolamine
NDRI (Bupropion) • AA (Clonidine) • CB1 (Surinabant)Alcohol dependence AD inhibitor (Disulfiram, Calcium carbimide) • mGluR (Acamprosate) • Opioid receptor antagonists (Naltrexone, Nalmefene) • Topiramate • AA (Clonidine) • BaclofenOpioid dependence Buprenorphine • Methadone • Levacetylmethadol • Dihydrocodeine • Dihydroetorphine • AA (Clonidine) • Lofexidine • Extended Release Morphine • Extended Release Hydromorphone • Baclofen • IbogaineStimulant dependence Benzodiazepine dependence Cocaine dependence Sedative-Hypnotic dependence GABAergics Receptor
ligandsReuptake
inhibitorsPlasmalemmalGAT inhibitorsCI-966 • Deramciclane • EF-1502 • Gabaculine • Guvacine • Nipecotic acid • NNC 05-2090 • SKF-89976A • SNAP-5114 • TiagabineEnzyme
inhibitorsGAD inhibitorsAllylglycineGABA-T inhibitors3-Hydrazinopropionic acid • Aminooxyacetic acid • Gabaculine • Isoniazid • Phenelzine • Phenylethylidenehydrazine • Sodium valproate • Valnoctamide • Valproate pivoxil • Valproate semisodium (Divalproex sodium) • Valproic acid • Valpromide • VigabatrinOthers Glutamate • GlutamineOthersCategories:- Amines
- Carboxylic acids
- Muscle relaxants
- Organochlorides
Wikimedia Foundation. 2010.