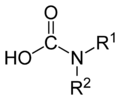
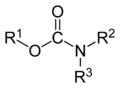
- Carbamic acid
-
Carbamic acid 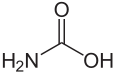
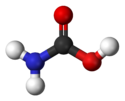 Carbamic acid
Carbamic acidIdentifiers CAS number 463-77-4 
PubChem 277 ChemSpider 271 
DrugBank DB04261 KEGG C01563 
MeSH Carbamic+acid ChEBI CHEBI:28616 
ChEMBL CHEMBL125278 
Jmol-3D images Image 1 - O=C(O)N
Properties Molecular formula CH3NO2 Molar mass 61.040 g/mol Related compounds Related compounds Dithiocarbamate
Carbonic acid
Urea
Ethyl carbamate acid (verify) (what is:
acid (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Carbamic acid is a compound that is unstable under normal circumstances. It is technically the simplest amino acid, though its instability (and the unique nature of the carboxyl-nitrogen bond) allows glycine to assume this title. Its importance is due more to its relevance in identifying the names of larger compounds. [1] Carbamic acid itself has not been synthesized or characterized by any experimental technique.[2]
The radical is called "carbamoyl". "Carbamoyltransferases" are transferase enzymes classified under EC number 2.1.3.
Carbamic acids are intermediates in the decomposition of carbamate protecting groups; the hydrolysis of an ester bond produces carbamic acid the evolution of carbon dioxide drives the deprotection reaction forward, yielding the unprotected amine.
Carbamates
Main article: CarbamateCarbamate is an ester of carbamic acid. Methyl carbamate is the simplest ester of carbamic acid.
Some esters have use as muscle relaxants,[3] while others are used as insecticides, for example aldicarb.[4]
See also
References
- ^ Thomas L. Lemke. (2003). Review of organic functional groups : introduction to medicinal organic chemistry. Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 63. ISBN 9780781743815. http://books.google.com/?id=a3L7eYbsyhkC&pg=PT24.
- ^ R.K. Khanna, M.H. Moore. (1998). "A 55". Carbamic acid: molecular structure and IR spectra (pii: S1386-1425(98)00228-5). Greenbelt, MD.: Elsevier. pp. 961–967. http://science.gsfc.nasa.gov/691/cosmicice/reprints/Carbamic.pdf.
- ^ ed. by John H. Block, John M. Beale. (2004). "Central Nervous System Depressant". Wilson and Gisvold's textbook of organic medicinal and pharmaceutical chemistry. Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 495. ISBN 9780781734813. http://books.google.com/?id=CeibVy3-LSMC&pg=PA495.
- ^ Risher, JF; Mink, FL; Stara, JF (1987). "The toxicologic effects of the carbamate insecticide aldicarb in mammals: a review". Environmental health perspectives 72: 267–81. doi:10.2307/3430304. JSTOR 3430304. PMC 1474664. PMID 3304999. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1474664.

This article about an organic compound is a stub. You can help Wikipedia by expanding it.