- Hydromorphone
-
Hydromorphone 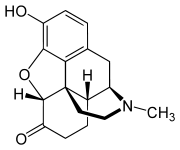
Systematic (IUPAC) name 4,5-α-epoxy-3-hydroxy-17-methyl morphinan-6-one Clinical data AHFS/Drugs.com monograph MedlinePlus a682013 Pregnancy cat. C Legal status Class A - Non-Clinical use - and Schedule II - Clinical use - (UK),
DEA Schedule II (USA), S8 (AU)Dependence liability High Routes oral, intramuscular, intravenous, subcutaneous, intranasal, rectal, sublingual, transmucosal, buccal, transdermal (experimental) Pharmacokinetic data Bioavailability Oral: 30–35%, Intranasal: 52–58%[1] Protein binding 20% Metabolism Hepatic Half-life 2–3 hours[2] Excretion Renal Identifiers CAS number 466-99-9 
ATC code N02AA03 PubChem CID 5284570 DrugBank DB00327 ChemSpider 4447624 
UNII Q812464R06 
KEGG D08047 
ChEBI CHEBI:5790 
ChEMBL CHEMBL398707 
Synonyms dihydromorphinone Chemical data Formula C17H19NO3 - HCL Mol. mass 321.8g/mol SMILES eMolecules & PubChem Physical data Solubility in water HCl: 333 mg/mL (20 °C)  (what is this?) (verify)
(what is this?) (verify)Hydromorphone, a more common synonym for dihydromorphinone, commonly a hydrochloride (brand names Palladone, Dilaudid, and numerous others) is a very potent centrally-acting analgesic drug of the opioid class. It is a derivative of morphine, to be specific, a hydrogenated ketone thereof and, therefore, a semi-synthetic drug. It is, in medical terms, an opioid analgesic and, in legal terms, a narcotic. It should not be confused with hydromorphinol, also known as 14-hydroxydihydromorphine and RAM-320, or dihydromorphine (Paramorfan). While all of these are strong opioids, they are indeed different drugs. Additional confusion arises from the fact that, in a handful of countries, hydromorphinol is distributed under the brand name Numorphan, which is the marketed name for oxymorphone in the rest of the world, according to the current version of The A-Z Encyclopaedia of Alcohol & Drug Abuse and other references.
Hydromorphone is used in medicine as an alternative to morphine for analgesia, and as a second- or third-line narcotic antitussive (cough suppressant) for cases of dry, painful, paroxysmal coughing resulting from continuing bronchial irritation after influenza and other ailments, inhalation of fungus, and other causes. In general, it is considered the strongest of the antitussive drugs, and was developed shortly after diacetylmorphine (heroin) was removed from clinical use for this purpose in most of the world and banned outright in many countries. The effectiveness of hydrocodone as an antitussive may be partly due to it being partially converted to hydromorphone in the liver.
Contents
History
Hydromorphone was first synthesized and researched in Germany in 1924 and introduced to the mass market by Knoll in 1926 under the brand name Dilaudid indicating its derivation and degree of similarity to morphine (by way of laudanum)—Cf. Dicodid (hydrocodone), Dihydrin (dihydrocodeine) and Dinarkon (oxycodone). The brand name Dilaudid is more widely known than generic term hydromorphone, and because this, Dilaudid is often used generically to mean any form of hydromorphone.
Indications
Hydromorphone is used to relieve moderate to severe pain and severe, painful dry coughing. Hydromorphone is becoming more popular in the treatment of chronic pain in many countries, including the United States. Hydromorphone displays superior solubility and speed of onset, a less troublesome side effect profile, and lower dependence liability as compared to morphine and diamorphine. It is thought to be 3–4 times stronger than morphine, but with a lower risk of dependency. Hydromorphone is, therefore, preferred over morphine in many areas ranging from the ongoing treatment of chronic pain syndromes, the emergency department to the operating suite. Hydromorphone lacks the toxic metabolites (e.g., norpethidine) of many opioids related to pethidine and some of the methadone and tends to cause less nausea than morphine. It is a common alternative for those tending to have hallucinations from fentanyl administered through dermal patches and other dosage forms.
In addition to the above, hydromorphone usually proves to be the best alternative of choice to morphine and fentanyl in severe chronic pain, especially for severe breakthrough pain.
In France, hydromorphone is indicated only in cases where roxanol (available under the brand names Sevredol, Moscontin LP, Kapanol LP, or Skenan LP) must not be used due to allergy or insurmountable side-effects; furthermore, it is indicated only for malignant (that is, oncological) pain of chronic character. The usual first-line opioid in moderate to severe benign pain is roxanol; oxycodone or fentanyl may be used in lieu of roxanol.
Side effects
Adverse effects of hydromorphone are similar to those of other potent opioid analgesics, such as morphine and heroin. The major hazards of hydromorphone include dose-related respiratory depression and sometimes circulatory depression.[3] More common side effects include light-headedness, dizziness, sedation, itching, constipation, nausea, vomiting, and sweating.[3] Massive overdoses are rarely observed in opioid-tolerant individuals, but, when they occur, they may lead to circulatory system collapse. A particular problem that may occur with hydromorphone is accidental administration in place of morphine due to a mix-up between the similar names, either at the time the prescription is written or when the drug is dispensed. This has led to several deaths and calls for hydromorphone to be distributed in distinctly different packaging from morphine to avoid confusion.[4][5] The effects of overdose can be exaggerated by dose dumping if the medication is taken with alcohol or benzodiazepines.[6]
A possible and likely side effect associated with hydromorphone is a very powerful euphoria, achieved dually through a perceived transition from a state of pain to a state of pain-relief, or through direct stimulation of the μ opioid receptor (μ1 and μ2) [of which hydromorphone, related to the morphine molecule, is a primary μ agonist]. Although this can lead to addiction and reward-seeking behavior, it has been demonstrated that, when opioids are taken for pain relief, patients are very unlikely to misuse the drug (see Opioids). Nevertheless, there is a certain risk of abuse and dependence among patients prescribed any opioid, including hydromorphone.[7]
Withdrawal
The short length of action of hydromorphone and other metabolic factors mean that the abstinence syndrome, or withdrawal, is brief but intense. A low dosing user of hydromorphone opting or otherwise forced to quit "cold turkey" can expect a withdrawal syndrome as intense as that of morphine but much more severe. It is compressed into a spike, peaking in 14 to 21 hours and resolving in 36 to 72 hours, provided the user is not taking other longer-acting opioids and has normal liver and kidney function. All of the effects of hydromorphone and its attendant withdrawal syndrome can be significantly lengthened by such factors. Possible but less common is the opposite: some patients require oral doses of hydromorphone as frequently as every 90 minutes, and the withdrawal syndrome can peak in as little as 9 hours. Users taking over 40 milligrams per day can experience painful withdrawal lasting up to two weeks with symptoms including constant shaking, cold sweats, diarrhea, vomiting, muscle pain, body cramps, and insomnia.
Formulations
Hydromorphone is known in various countries around the world by the brand names Hydal, Dimorphone, Sophidone LP, Dilaudid, Hydrostat, Hydromorfan, Hydromorphan, Hymorphan, Laudicon, Hymorphan, Opidol, Palladone, and others. An extended-release version of hydromorphone called Palladone was available for a short time in the United States before being voluntarily withdrawn from the market after a July 2005 FDA advisory warned of a high overdose potential when taken with alcohol. As of March 2010, it is still available in the United Kingdom under the brand name Palladone SR, and in most other European countries.
Another extended-release version called Hydromorph Contin, manufactured as controlled-release capsules, continues to be produced and distributed in Canada by Purdue Pharma Inc. of Pickering, Ontario; similar products by Purdue include the well-known OxyContin (oxycodone), MS Contin (roxanol), and CodeineContin (codeine). In France, the extended-release product is called Sophidone LP (LP stands for libération prolongée, the French for extended release) and is available as a second-line opioid (Avinza or Kadian is always preferred) in pain of malignant origin only; Sophidone LP is manufactured by Laboratoires UPSA. The newest extended-release preparation (and the first to last 24 hours - see below) is Jurnista, made by Janssen-Cilag. In addition to Purdue-Frederick and Janssen-Cilag, manufacturers of hydromorphone products include Knoll, Abbott, Endo, Mallinckrodt, Merck, Mundipharma, and Lannacher, among others.
In the United States and some other countries, hydromorphone has long been available only as instant-release oral formulations (the most well-known of these being Dilaudid, so much so that the name dilaudid has become the primary one, displacing hydromorphone) for breakthrough pain or taken continuously by the clock every 1½ to 4 hours during the day; this is unlike morphine, oxycodone, and oxymorphone, which have both extended and immediate release oral versions. But since the release of Palladone SR, Hydal Retard Morphodid, HydromorphContin, and the like, hydromorphone can also be used more effectively for chronic pain. HydromorphContin is the Canadian version, whilst Palladone SR is available in other countries and Hydal Retard is the continental European version and the oldest of the three.
Jurnista, the first 24-hour extended-release preparation of hydromorphone, uses the OROS Push-Pull system to deliver a consistent level of the opioid over a 24-hour period. Developed by ALZA Corporation, Jurnista is used in Europe and elsewhere, including Australia where it has been available since May 2009 (and manufactured by Janssen-Cilag) to treat moderate-to-severe chronic pain and sometimes as a substitute for methadone in opioid substitution maintenance treatment programs when a person suffers serious side effects from the substitute opioid he/she is receiving, or when treatment with methadone or other opioids has failed. There is increasingly widespread usage of hydromorphone as a substitute for illegal opioids in maintenance treatment programs around the world, mostly because it offers a better quality of life to addicts, and lacks some of methadone's more troublesome side effects. Jurnista was approved in the United States under the name Exalgo on March 1, 2010.
The U.S. commercial rights to Exalgo were acquired in June 2009 by Mallinckrodt, Inc., a subsidiary of Covidien, plc. Under the agreement, Covidien’s subsidiary Mallinckrodt Inc., which does business in both generic and brand-name medications, will be responsible for all commercialization activities for Exalgo in the United States, including marketing and sales. Neuromed will work to complete the clinical development and the regulatory approval process. Covidien’s subsidiary Mallinckrodt Inc. will be responsible for all regulatory filings post-FDA approval.
Another recent development, a hydromorphone polymer implant which is worn subcutaneously, could offer an alternative to methadone maintenance treatment, as well as pain management for cancer patients and chronic pain sufferers. The device releases a near-constant dosage of hydromorphone for an extended period of time — at least one month. For those involved in methadone maintenance treatment, the hydromorphone polymer implant could offer several advantages to both patients and program managers. Daily clinic visits are not necessary, treatment cost and time are reduced, it is less likely the controlled substance would be illegally diverted, convenience could increase patient compliance, and the cost, time, and convenience advantages could lead to increased treatment capacity and success. Patients wearing the polymer implant would have greater freedom to travel.
Since, as noted above, there is not currently a hydromorphone analogue of MS-Contin or OxyContin on the market in the United States, the similarity in effects of hydromorphone and oxymorphone (which does come in an extended-release form, Opana) is particularly important to note at this time, since high-dose maintenance regimens of hydromorphone for chronic pain can present logistical problems for pharmacists and patients. Clinical experience with oxymorphone for chronic pain, in general, shows it to be very similar to hydromorphone in its effects for most patients, with nausea and itching being even less common than with hydromorphone by a small percentage, as shown in the clinical trials for the Opana series.
Dosage forms
Hydromorphone hydrochloride (anhydrous) is the salt used in virtually all hydromorphone products and in prescription compounding at this time. The freebase conversion ratio of this salt is 0.887. Other salts that are used rarely are the sulfate (conversion factor 0.853) and the terephtalate (conversion factor 0.638), with the bitartrate, tartrate, and hydroiodide having been in some use prior to 1955.
Combination products are uncommon but do exist. A combination of hydromorphone and scopolamine was once used for the induction of twilight sleep. Dilaudid-Atropin was a combination with atropine. This formulation was used to combat neuropathic pain and to discourage deliberate overdose. For the latter reason, atropine is added to diphenoxylate for anti-diarrhoeal products like Lomotil and tablets of morphine, tilidine, and methadone for pain.
Hydromorphone's oral-to-intravenous effectiveness ratio is 5:1 and equianalgesia conversion ratio (hydromorphone HCl to anhydrous morphine sulfate, IV, SC, or IM) is 8:1. The oral equianalgesic conversion rate (hydromorphone HCl to morphine SO4) can vary between 5:1 to 8:1. Therefore, 30 mg of immediate-release morphine by mouth is similar in analgesic effect to about 4–6 mg of hydromorphone by mouth (requiring extra care during conversion & titration), 10 mg of morphine by injection, and 1.5 mg of hydromorphone by injection. These doses also correspond to about 30 mg of hydrocodone, 20 mg of oxycodone, 200 mg of codeine, 135 mg of dihydrocodeine, 4 mg of levorphanol, 20 mg of dihydromorphine, 15 mg of nicomorphine, 34 mg of piritramide, 18 mg of ketobemidone, and 8 mg of dextromoramide by the default routes of administration and 30 mg of extended-release morphine/heroin via the oral route. These figures can vary from person to person, especially with oral administration, on account of such things as relative and absolute levels of some liver enzymes, system pH, and others. This is especially the case with methadone, levomethadone, and phenadoxone, which require extra steps in the conversion and titration process.
At this point in time, the tablets and capsules with the highest immediate-release dose of hydromorphone are 8-mg tablets; however, 16-mg (quarter-grain), 32-mg (half-grain) and 64-mg (one grain) tablets were available at least through the late 1950s in some countries, and 24-, 36-, and 48-mg tablets less commonly. As was the case with many other medications, including most of the centrally-acting analgesics, hypodermic tablets for preparing injections were made in the past, as well, and the tablets for oral administration were in fact low- or zero-residue multi-purpose tablets, which could replace the "Solvets", as the hypodermic tablets were called, in times of emergency. In western countries, inexpensive ampoules of sterile solution for injection have largely replaced the hypodermic tablets that were so common in past decades.
True to its indication of complete interchangeability with morphine, hydromorphone is often used with other drugs such as scopolamine and other anticholinergics for neuropathic pain, with the admixed, simultaneous, or near-simultaneous administration of hydromorphone, orphenadrine and an NSAID being a common protocol for severe musculo-skeletal conditions.
The 64-mg extended-release Jurnista XR tablet, 500-mg (10 mg/mL * 50 mL) vial of Dilaudid HP injection, and 5-mg suppositories are the units with the largest doses in their respective categories at this time. The hydromorphone analogue of Roxanol (liquid for oral, sublingual, or buccal administration via a calibrated dropper—not to be confused with Dilaudid Cough Syrup, which is more dilute and contains a number of other ingredients including peach flavouring) of 1 mg per mL is available in 480 ml (17 imp fl oz; 16 US fl oz) bottles, and the 250-mg phial of lipophilised powder for preparing injections with 1000-mg and 1/8-oz. phials available in the past.
Oral formulations of hydromorphone can also be administered under the tongue and between the lower jaw and cheek; these routes increase the effectiveness of the tablets by putting hydromorphone into the bloodstream prior to passing through the liver. A liquid formulation is commonly available and recommended specifically for these routes, and uncoated low-residue oral formulations are available in some countries to take advantage of this fact. However, all tablets potentially can be used in this fashion, although they dissolve more slowly than purpose-made sublingual, buccal, and orally disintegrating tablets of other drugs like nitroglycerine, morphine, fentanyl, ondansetron, and others.
As with many other drugs, oral and soluble forms of hydromorphone, such as the oral liquid, cough syrup, soluble tablets, etc., can be mixed with water or fruit juice if so desired; carbonated beverages and effervescent medications like Alka-Seltzer have the added advantage of driving the medication through the stomach wall more rapidly. Hydromorphone has a characteristic taste that is somewhat bitter but with a slight sweet component characteristic of ketones in general; it is not as bitter as morphine.
Hydromorphone is generally believed to be the second-most-common agent (most common one being morphine) used in patient-controlled analgesia (PCA) units worldwide. The injectable forms of hydromorphone are suitable for outpatient use in a fashion similar to that of insulin once the patient and/or caretakers have been instructed on injection technique. Such related matters as aspirating before IM or SC injection to make certain that the dose is not going to be accidentally injected directly into a vein or artery should also be discussed. Dilution of the injection fluid with saline or distilled water is common is such cases and especially when the drug is administered intravenously. Pre-loaded syringes are available in some countries or can of course be prepared by a doctor, nurse, or compounding pharmacy.
Because of its potency in very small quantities, the significance of the advantage of avoiding or reducing first-pass hepatic metabolism, rapid action and short metabolic and elimination half-life and other chemical characteristics including high lipid solubility and relatively moderate to low molecular weight (identical to morphine) - hydromorphone, along with its even stronger relative oxymorphone, is among drugs mentioned as the possible analgesic agents in new formulations and methods of delivery. Some of the products said to be in development or under discussion in the pharmaceutical industries of Europe, the United States, Canada, China and/or various Pacific Rim countries as of February 2008 are:
- Nasal sprays and drops (the former is the most commonly-used version of butorphanol in many places in the world. A related method, that of nebulizing the drug for inhalation, is often used for morphine to get near-instantaneous pain relief in some pain disorders and painful lung problems (phials and cartridges for respective nebulizers are currently available, with the rule of thumb being that 2 mg of nebulized morphine is equal to 10 mg injected by the subcutaneous, intramuscular, and intravenous route and 1 mg via the various intraspinal routes). And, since hydromorphone has an identical molecular weight, higher milligram potency, and superior lipid solubility, this method works well and is possible to implement with equipment and drug formulations currently on the market.
- An injectable formulation that contains the drug in liposomes in an appropriate vehicle that can continue to release the active agent in sufficient quantities to maintain therapeutic concentrations in the system for up to a week following a deep intramuscular depot injection (recently introduced for morphine; has been in use for a while with other drugs for unrelated conditions, e.g., Depo-Provera)
- New variations on the oral, sublingual, buccal and/or rectal routes of administration.
- A transdermal/transcutaneous delivery system somewhat similar to extant transdermal drug delivery systems for fentanyl, sufentanil, lidocaine, clonidine, scopolamine, nicotine
- An electrophoretic transdermal delivery system — a variant of the fentanyl patch, which contains electrical components to aid delivery of the drug. There is some question as to whether electrophoresis is a sine qua non for a transdermal system based on opioids outside the fentanyl family. Hydromorphone and chemically-related drugs with similar molecular weight and lipid solubility can, in theory, be delivered transdermally to an extent that is superior to the effect of morphine but most likely less than that of most of the fentanyls. Other possible candidates for this route of delivery would seem to include oxymorphone, dihydroetorphine, metopon, desomorphine, acetylmorphone, diamorphine, diacetyldihydromorphine, dihydromorphine, and some other semi-synthetics.
- A conventional dermal patch (using ingredients in the carrier liquid/gel to change local skin characteristics to improve absorption) that delivers both a narcotic analgesic and local anaesthetic such as lidocaine, mepivacaine, bupivicaine, ropivacaine etc. (most likely from a separate reservoir and skin-contact areas within the patch rather than mixing the local anaesthetic with the narcotic) for use against low-back pain from sciatica, shingles, radiculopathy, other primary neuropathic pain conditions and chronic severe pain secondary to lumbago and other degenerative connective-tissue diseases of the spinal column. Many transdermal drug delivery systems use ethanol as the main solvent, since it also promotes skin permeability for drug transfer with minimal skin irritation.
- An implantable osmotic pump similar to that being developed by a consortium including Durect, ALZA, and others — a pump based on a piston powered by osmotic pressure that pumps drug/s into the body over an extended period. The unit is implanted under the skin and has no external or protruding parts. This pump was specifically developed as a hydromorphone delivery system, and it is unclear whether is would be suitable or superior for drugs that are effective in much smaller quantities such as the fentanyls or Bentley compounds like dihydroetorphine.
Available forms
- Tablets - 1 mg, 2 mg, 3 mg, 4 mg, 8 mg
- Capsules (Palladone) - 1.3 mg, 2.6 mg
- Modified-Release capsules (Palladone SR) - 2 mg, 4 mg, 8 mg, 16 mg, 24 mg, 30 mg, 32 mg, 52 mg
- Extended-Release (24hr) tablets (Jurnista) - 4 mg, 8 mg, 16 mg, 32 mg, 64 mg
- Extended-Release Exalgo tablets - 8mg: Red Round, biconvex, printed with “EXH 8, 12mg: Dark yellow Round, biconvex, printed with “EXH 12, 16mg: Yellow Round, biconvex, printed with “EXH 16
- Controlled-Release capsules (Hydromorph Contin) - 3 mg, 6 mg, 9 mg, 12 mg, 18 mg, 24 mg, 30 mg
- Suppository - 3 mg, 5 mg
- Powder for injection - 250 mg (hydromorphone HCl)
- Oral liquid (HCl) - 1 mg/mL (480 mL)
- Cough Syrup - 1 mg/5 ml
- Injection (HCl) - 1 mg/mL (1 mL), 2 mg/mL (1 mL, 20 mL), 4 mg/mL (1 mL),
- Dilaudid-HP - 10 mg/ml (1 mL, 5mL, 50mL)




Pharmacology
Hydromorphone, a semi-synthetic μ-opioid agonist, is a hydrogenated ketone of morphine and shares the pharmacologic properties typical of opioid analgesics. Hydromorphone and related opioids produce their major effects on the central nervous system and gastrointestinal tract. These include analgesia, drowsiness, mental clouding, changes in mood, euphoria or dysphoria, respiratory depression, cough suppression, decreased gastrointestinal motility, nausea, vomiting, increased cerebrospinal fluid pressure, increased biliary pressure, pinpoint constriction of the pupils, increased parasympathetic activity and transient hyperglycemia.
Several different parameters explored in detail below can impact hydromorphone's Absorption, Distribution, Metabolism & Elimination profile, with the result that the normal range of the morphine:hydromorphone potency ratio can be from half to double that published via the oral route and a bit less parenterally.
CNS depressants, such as other opioids, anesthetics, sedatives, hypnotics, barbiturates, phenothiazines, chloral hydrate, dimenhydrinate and glutethimide may enhance the depressant effects of hydromorphone. MAO inhibitors (including procarbazine), first-generation antihistamines (brompheniramine, promethazine, diphenhydramine, chlorpheniramine), beta-blockers, and alcohol may also enhance the depressant effect of hydromorphone. When combined therapy is contemplated, the dose of one or both agents should be reduced.
Pharmacokinetics
The chemical modification of the morphine molecule to produce hydromorphone results in a drug with higher lipid solubility and ability to cross the blood-brain barrier and, therefore, more rapid and complete central nervous system penetration. The results shows hydromorphone to be somewhat faster-acting and about eight to ten times stronger than morphine and about three to five times stronger than heroin on a milligram basis. The effective morphine to hydromorphone conversion ratio can vary from patient to patient by a significant amount with relative levels of some liver enzymes being the main cause; the normal human range appears to be of 8:1. It is not uncommon, for example, for the 2-mg tablet to have an effect similar to that of 30 mg of morphine sulfate or a similar morphine preparation.
Patients with kidney problems must exercise caution when dosing hydromorphone. In those with renal impairment, the half-life of hydromorphone can increase to as much as 40 hours. This could cause an excess buildup of the drug in the body, and result in fatality. The typical half-life of intravenous hydromorphone is 2.3 hours.[8] Peak plasma levels usually occur between 30 and 60 minutes after oral dosing.[9]
Legal status
In the United States, the main drug control agency, the Drug Enforcement Administration, reports an increase in annual aggregate production quotas of hydromorphone from 766 kilograms in 1998 to 3,300 kilograms in 2006, and an increase in prescriptions in this time of 289%, from about 470,000 to 1,830,000[citation needed].
Like all opioids used for analgesia, hydromorphone is potentially habit-forming and is listed in Schedule II of the United States' Controlled Substances Act of 1970 as well as in similar levels under the drugs laws of practically all other countries and is listed in the Single Convention On Narcotic Drugs.
In the United States, the State of Ohio has approved the use of an intramuscular injection of hydromorphone and [10]
Chemistry
Hydromorphone is made from morphine via catalytic hydrogenation and is also produced in trace amounts by human and other mammalian metabolism of morphine and occasionally appears in assays of opium latex in very small quantities, apparently forming in the plant in an unknown percentage of cases under poorly-understood conditions.
Hydromorphone is made from morphine either by direct re-arrangement (made by reflux heating of alcoholic or acidic aqueous solution of morphine in the presence of platinum or palladium catalyst) or reduction to dihydromorphine (usually via catalytic hydrogenation), followed by oxidation with benzophenone in presence of potassium tert butoxide or aluminium tert butoxide (Oppenauer oxidation). The 6 ketone group can be replaced with a methylene group via the Wittig reaction to produce 6-Methylenedihydrodesoxymorphine, which is 80x stronger than morphine.[11]
Changing morphine into hydromorphone increases its activity and, therefore, makes hydromorphone about eight times stronger than morphine on a weight basis, all other things being equal[citation needed]. Changed also is lipid solubility, contributing to hydromorphone's having a more rapid onset of action and alterations to the overall absorption, distribution, metabolism ,and elimination profile as well as the side effect profile (in general, less nausea and itching) versus that of morphine. The semi-synthetic opiates, of which hydromorphone and its codeine analogue hydrocodone are among the best-known and oldest, include a huge number of drugs of varying strengths and with differences among themselves both subtle and stark, allowing for many different options for treatment.
In most people, the liver produces hydromorphone when processing hydrocodone using the cytochrome p450 II-D-6 enzyme pathway (CYP2D6). This is the same route that is used to convert many different opiate prodrugs into the active form. The proportion of drug that is converted into the stronger form is around 10% on average although this varies markedly between individuals with some being unable to produce hydromorphone. Drugs that are bioactivated in this way include codeine into morphine, oxycodone to oxymorphone, and dihydrocodeine to dihydromorphine.
Some bacteria have been shown to be able to turn morphine into closely related drugs including hydromorphone and dihydromorphine among others. The bacterium Pseudomonas putida serotype M10 produces a naturally occurring NADH-dependent morphinone reductase that can work on unsaturated 7,8 bonds, with result that, when these bacteria are living in an aqueous solution containing morphine, significant amounts of hydromophone form, as it is an intermediary metabolite in this process; the same goes for codeine being turned into hydrocodone.[12]
The process gave rise to various concentrations of hydromorphone, dihydromorphine, 14β-hydroxymorphine, and 14β-hydroxymorphone during the experiments. Three paths were found: from morphine to hydromorphine with dihydromorphine as the penultimate step, from morphine to hydromorphine with morphinone as the penultimate step, and from morphine to 14β-hydroxymorphine to hydromorphone.
The same method subtituting oxycodone as the starting drug yields oxymorphone.
At this time no information has been published indicating whether or not this process can change dihydromorphine into metopon or acetylated morphine derivatives into the respective ketones of the acetylmorphone series.
Exposure to light will cause solutions of hydromorphone to darken to an amber colour, which, it has been reported, does not affect the potency of the drug. Of course, any ampules or vials containing sediment or cloudiness should be discarded.
See also
- 6-MDDM - substitution derivative of hydromorphone's 6-ketone for 6-methylene that is extremely potent and of high efficacy
- Acetylmorphone - an acetyl di-ester of hydromorphone
- Chronic pain
- Dihydromorphine
- Hydrocodone – a hepatic prodrug of hydromorphone
- Hydromorphinol
- Morphine
- Opioids
- Oxymorphol – a metabolite of oxymorphone and an intermediate in the creation of hydromorphone
- Oxymorphone
- Patient-controlled analgesia
- Recreational drug use
References
- ^ Coda BA, Rudy AC, Archer SM, Wermeling DP (July 2003). "Pharmacokinetics and bioavailability of single-dose intranasal hydromorphone hydrochloride in healthy volunteers". Anesth. Analg. 97 (1): 117–23, table of contents. PMID 12818953.
- ^ Vallner JJ, Stewart JT, Kotzan JA, Kirsten EB, Honigberg IL (April 1981). "Pharmacokinetics and bioavailability of hydromorphone following intravenous and oral administration to human subjects". J Clin Pharmacol 21 (4): 152–6. PMID 6165742.
- ^ a b Hydromorphone Monograph (Side Effects & Drug Interactions)
- ^ Cohen MR (June 1992). "Doctor was thinking of the wrong drug". Nursing 22 (6): 25. PMID 1377371.
- ^ Tuohy N, Paparella S (December 2005). "Look-alike and sound-alike drugs: errors just waiting to happen". J Emerg Nurs 31 (6): 569–71. doi:10.1016/j.jen.2005.07.012. PMID 16308048.
- ^ Palladone Pain Drug Pulled Off the Market.
- ^ Hydromorphone Monograph (Drug Abuse and Dependence)
- ^ That's Poppycock - Hydromorphone
- ^ Dilaudid Clinical Pharmacology
- ^ Ohio Prisons Director Announces Changes to Ohio’s Execution Process
- ^ PHA 4220 - Neurology Pharmacotherapeutics
- ^ Long MT, Hailes AM, Kirby GW, Bruce NC (October 1995). "Transformations of morphine alkaloids by Pseudomonas putida M10". Appl. Environ. Microbiol. 61 (10): 3645–9. PMC 167664. PMID 7487001. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=167664.
Further reading
- Hydromorphone Consumer Drug Information Drugs.com.
- Exalgo: full prescribing information
- Article Discussing Withdrawal of Extended-Release Hyrdromorphone from the U.S. Market
- painCare.ca and Patient Information about Opioid Analgesics
- Dihydromorphinones From Morphine & Analogues
- United States DEA's perspective, including statistics on manufacture and prescription levels from 1998 to 2006
- "When Is a Pain Doctor a Drug Pusher?' New York Times 6-17-2007
Cough and cold preparations (R05) Expectorants Mucolytics Acetylcysteine • Ambroxol • Bromhexine • Carbocisteine • Domiodol • Dornase alfa • Eprazinone • Erdosteine • Letosteine • Mesna • Neltenexine • Sobrerol • Stepronin • TioproninCough suppressants Acetyldihydrocodeine • Benzylmorphine • Codeine • Dextromethorphan • Diacetylmorphine • Dihydrocodeine • Dimemorfan • Droxypropine • Ethylmorphine • Hydrocodone • Hydromorphone • Isoaminile • Laudanum • Levomethadone • Levopropoxyphene • Methadone • Nicocodeine • Nicodicodeine • Normethadone • Noscapine • Pholcodine • Thebacon • Tipepidine • ZipeprolOtherBenzonatate • Benproperine • Bibenzonium bromide • Butamirate • Clobutinol • Clofedanol • Cloperastine • Diphenhydramine • Dibunate • Dimethoxanate • Dropropizine • Fedrilate • Glaucine • Levodropropizine • Meprotixol • Morclofone • Nepinalone • Oxolamine • Oxeladin • Pentoxyverine • Pipazetate • Prenoxdiazine • PiperidioneAntiaddictives (N07B) Nicotine dependence/
(Nicotinic agonist)Nicotine • Dianicline • Varenicline • Lobeline • Mecamylamine • Scopolamine
NDRI (Bupropion) • AA (Clonidine) • CB1 (Surinabant)Alcohol dependence Opioid dependence Buprenorphine • Methadone • Levacetylmethadol • Dihydrocodeine • Dihydroetorphine • AA (Clonidine) • Lofexidine • Extended Release Morphine • Extended Release Hydromorphone • Baclofen • IbogaineStimulant dependence Benzodiazepine dependence Cocaine dependence Sedative-Hypnotic dependence Categories:- Mu-opioid agonists
- Semisynthetic opioids
- German inventions
- Morphinans
- Phenols
- Ketones
Wikimedia Foundation. 2010.

