- Aspirin
-
Aspirin 
Systematic (IUPAC) name 2-acetoxybenzoic acid Clinical data AHFS/Drugs.com monograph MedlinePlus a682878 Pregnancy cat. C(AU) D(US) Legal status Unscheduled (AU) GSL (UK) OTC (US) Routes Most commonly oral, also rectal. Lysine acetylsalicylate may be given IV or IM Pharmacokinetic data Bioavailability Rapidly and completely absorbed Protein binding 99.6% Metabolism Hepatic Half-life 300–650 mg dose: 3.1–3.2 h
1 g dose: 5 h
2 g dose: 9 hExcretion Renal Identifiers CAS number 50-78-2 
ATC code A01AD05 B01AC06, N02BA01 PubChem CID 2244 DrugBank DB00945 ChemSpider 2157 
UNII R16CO5Y76E 
KEGG D00109 
ChEBI CHEBI:116450 
ChEMBL CHEMBL25 
Synonyms 2-acetyloxybenzoic acid
acetylsalicylate
acetylsalicylic acid
O-acetylsalicylic acidChemical data Formula C9H8O4 Mol. mass 180.157 g/mol SMILES eMolecules & PubChem Physical data Density 1.40 g/cm³ Melt. point 135 °C (275 °F) Boiling point 140 °C (284 °F) (decomposes) Solubility in water 3 mg/mL (20 °C)  (what is this?) (verify)
(what is this?) (verify)Aspirin (USAN), also known as acetylsalicylic acid (/əˌsɛtəlˌsælɨˈsɪlɨk/ ə-set-əl-sal-i-sil-ik; abbreviated ASA), is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It was discovered by Arthur Eichengrun, a chemist with the German company Bayer. [1]
Salicylic acid, the main metabolite of aspirin, is an integral part of human and animal metabolism. While much of it is attributable to diet, a substantial part is synthesized endogenously.[2]
Aspirin also has an antiplatelet effect by inhibiting the production of thromboxane, which under normal circumstances binds platelet molecules together to create a patch over damaged walls of blood vessels. Because the platelet patch can become too large and also block blood flow, locally and downstream, aspirin is also used long-term, at low doses, to help prevent heart attacks, strokes, and blood clot formation in people at high risk of developing blood clots.[3] It has also been established that low doses of aspirin may be given immediately after a heart attack to reduce the risk of another heart attack or of the death of cardiac tissue.[4][5]
The main undesirable side-effects of aspirin taken by mouth are gastrointestinal ulcers, stomach bleeding, and tinnitus, especially in higher doses. In children and adolescents, aspirin is no longer indicated to control flu-like symptoms or the symptoms of chickenpox or other viral illnesses, because of the risk of Reye's syndrome.[6]
Aspirin is part of a group of medication called nonsteroidal anti-inflammatory drugs (NSAIDs), but differs from them in the mechanism of action. Though it, and others in its group called the salicylates, have similar effects (antipyretic, anti-inflammatory, analgesic) to the other NSAIDs and inhibit the same enzyme cyclooxygenase, aspirin (but not the other salicylates) does so in an irreversible manner and, unlike others, affect more the COX-1 variant than the COX-2 variant of the enzyme.[7] For example, NSAIDs' antiplatelet effects normally last in the order of hours, whereas aspirin's effects last for days (until the body replaces the suppressed platelets). Hence, when physicians tell patients to stop taking NSAIDs, they usually imply aspirin as well.
Today, aspirin is one of the most widely used medications in the world, with an estimated 40,000 tonnes of it being consumed each year.[8] In countries where Aspirin is a registered trademark owned by Bayer, the generic term is acetylsalicylic acid (ASA).[9]
Contents
Medical uses
Aspirin is used for the treatment of a number of conditions including: fever, pain, rheumatic fever, inflammatory diseases such as rheumatoid arthritis, pericarditis, and Kawasaki disease.[10] It is used in the prevention of transient ischemic attacks, strokes, heart attacks, pregnancy loss, and cancer.[10]
Pain
In general, aspirin works well for dull, throbbing pain; it is ineffective for pain caused by most muscle cramps, bloating, gastric distension, and acute skin irritation.[11] The most studied example is pain after surgery, such as tooth extraction, for which the highest allowed dose of aspirin (1 g) is equivalent to 1 g of paracetamol (acetaminophen), 60 mg of codeine, or 5 mg of oxycodone. A combination of aspirin and caffeine, in general, affords greater pain relief than aspirin alone. Effervescent aspirin alleviates pain much faster than aspirin in tablets (15–30 min vs. 45–60 min).[12]
Nevertheless, as a postsurgery painkiller, aspirin is inferior to ibuprofen and has higher gastrointestinal toxicity. The maximum dose of aspirin (1 g) provides weaker pain relief than an intermediate dose of ibuprofen (400 mg), and this relief does not last as long.[12] A combination of aspirin and codeine may have a slightly higher analgesic effect than aspirin alone; however, this difference is not clinically meaningful.[13] It appears ibuprofen is at least equally, and possibly more, effective than this combination.[14]
According to a 1998 meta-analysis of clinical trials for menstrual pain, aspirin demonstrated higher efficacy than placebo, but lower than ibuprofen or naproxen, although maximum doses of aspirin were never used in these trials. The authors concluded ibuprofen has the best risk-benefit ratio.[15]
Aspirin did not ease pain during cycling exercise,[16] while caffeine was very effective.[17][18] Likewise, aspirin, codeine, or paracetamol was not better than placebo for muscle soreness after exercise.[19]
Headache
Aspirin is a first-line drug in the treatment of migraine, bringing relief in 50–60% of the cases.[20] When used at a high dose of 1000 mg (as compared to 275–325 mg when used as a pain killer or 81 mg as an antiplatelet therapy), no significant differences were seen as compared to triptan medication, sumatriptan (Imitrex)[21] and other painkillers such as paracetamol (acetaminophen)[22] or ibuprofen.[23] The combination of aspirin, paracetamol (acetaminophen) and caffeine (as found in the OTC brand Excedrin) is even more potent. For the treatment of migraine headache, this formulation works better than any of its three components taken separately,[22] better than ibuprofen[24] and better than sumatriptan. As with all other medications for migraine, it is recommended to take aspirin at the first signs of the headache, and it is the way these medications were used in the comparative clinical trials.[25]
Aspirin alleviates pain in 60–75% of patients with episodic tension headaches.[26][27] It is equivalent to paracetamol (acetaminophen) in that respect, except for the higher frequency of gastrointestinal side-effects.[27] Comparative clinical trials indicated metamizole and ibuprofen may relieve pain faster than aspirin, although the difference becomes insignificant after about two hours. The addition of caffeine in a dose of 60–130 mg to aspirin increases the analgesic effect in headache.[26][28] The combination of aspirin, paracetamol (acetaminophen) and caffeine is still more effective, but at the cost of more stomach discomfort, nervousness and dizziness.[29]
There is some evidence low-dose asprin has benefit for reducing the occurrence of migraines in susceptible individuals.[30][31][32][33]
Prevention of heart attacks and strokes
There are two distinct uses of aspirin for prophylaxis of cardiovascular events: primary prevention and secondary prevention. Primary prevention is about decreasing strokes and heart attacks in the general population of those who have no diagnosed heart or vascular problems. Secondary prevention concerns patients with known cardiovascular disease.[34]
Low doses of aspirin are recommended for the secondary prevention of strokes and heart attacks. For both males and females diagnosed with cardiovascular disease, aspirin reduces the chance of a heart attack and ischaemic stroke by about a fifth.[citation needed] This translates to an absolute rate reduction from 8.2% to 6.7% of such events per year for people already with cardiovascular disease.[citation needed] Although aspirin also raises the risk of hemorrhagic stroke and other major bleeds by about twofold, these events are rare, and the balance of aspirin's effects is positive. Thus, in secondary prevention trials, aspirin reduced the overall mortality by about a tenth.[34]
For persons without cardiovascular problems, the benefits of aspirin are unclear. In the primary prevention trials, aspirin decreased the overall incidence of heart attacks and ischaemic strokes by about a tenth. However, since these events were rare, the absolute reduction of their rate was low: from 0.57% to 0.51% per year. In addition, the risks of hemorrhagic strokes and gastrointestinal bleeding almost completely offset the benefits of aspirin. Thus, in the primary prevention trials, aspirin did not change the overall mortality rate.[34] Further trials are in progress[update].[34]
The expert bodies diverge in their opinions regarding the use of aspirin for primary prevention, such as can be accomplished by including aspirin in a polypill for the general population. The US Government Preventive Services Task Force recommended making individual, case by case choices based on the estimated future risk and patients' preferences.[35][36] On the other hand, Antithrombotic Trialists’ Collaboration argued such recommendations are unjustified, since the relative reduction of risk in the primary prevention trials of aspirin was same for persons in high- and low-risk groups and did not depend on the blood pressure. The Collaboration suggested statins as the alternative and more effective preventive medication.[34]
Coronary and carotid arteries, bypasses and stents
The coronary arteries supply blood to the heart. Aspirin is recommended for one to six months after placement of stents in the coronary arteries and for years after a coronary artery bypass graft.
The carotid arteries supply blood to the brain. Patients with mild carotid artery stenosis benefit from aspirin; it is recommended after a carotid endarterectomy or carotid artery stent.
After vascular surgery of the lower legs using artificial grafts that are sutured to the arteries to improve blood supply, aspirin is used to keep the grafts open because it serves as type of blood thinner, reducing the likelihood of clots forming.
Other uses
Although aspirin has been used to combat fever and pains associated with common cold for more than 100 years, its efficacy in this role was only recently confirmed in controlled clinical trials on adults. One gram of aspirin, on average, reduced the oral body temperature from 39.0 °C (102.2 °F) to 37.6 °C (99.7 °F) after three hours. The relief began after 30 minutes, and after six hours, the temperature still remained below 37.8 °C (100.0 °F). Aspirin also helped with "achiness", discomfort, and headache,[37] and with sore throat pain, for those who had it.[38] The effects of aspirin were indistinguishable from those obtained using paracetamol in any respect, except for, possibly, a slightly higher incidence of sweating and gastrointestinal side-effects.[37]
Fever and joint pain of acute rheumatic fever respond extremely well, often within three days, to high doses of aspirin. The therapy usually lasts for one to two weeks; and only in about 5% of the cases it has to continue for longer than six months. After fever and pain have subsided, the aspirin treatment is unnecessary, as it does not decrease the incidence of heart complications and residual rheumatic heart disease.[39] In addition, the high doses of aspirin used caused liver toxicity in about 20% of the treated children,[40][41] who are the majority of rheumatic fever patients, and increased the risk of their developing Reye's syndrome.[39] Naproxen was shown to be as effective as aspirin and less toxic; due to the limited clinical experience, however, naproxen is recommended only as a second-line treatment.[39][42]
Along with rheumatic fever, Kawasaki disease remains one of the few indications for aspirin use in children, although even this use has been questioned by some authors.[43] In the United Kingdom, the only indications for aspirin use in children and adolescents under 16 are Kawasaki disease and prevention of blood clot formation.
Aspirin is also used in the treatment of pericarditis, coronary artery disease, and acute myocardial infarction.[44][45][46]
Taking aspirin before air travel in cramped conditions has been suggested to decrease the risk of deep-vein thrombosis (DVT). The reason for taking aspirin is the long period of sitting without exercise, not air travel itself. A large, randomized, controlled trial in 2000 of aspirin against placebo in 13,000 patients with hip fractures found "a 29% relative risk reduction in DVT with 160 mg of aspirin taken daily for five weeks. Although there are obvious problems with extrapolating the data to long-distance travelers, this is the best evidence we could find to justify aspirin use".[47]
Experimental
Aspirin has been theorized to reduce cataract formation in diabetic patients, but one study showed it was ineffective for this purpose.[48] The role of aspirin in reducing the incidence of many forms of cancer has also been widely studied. In several studies, its use did not reduce the incidence of prostate cancer.[49][50] Its effects on the incidence of pancreatic cancer are mixed; one study published in 2004 found a statistically significant increase in the risk of pancreatic cancer among women,[51] while a meta-analysis of several studies, published in 2006, found no evidence aspirin or other NSAIDs are associated with an increased risk for the disease.[52] The drug may be effective in reduction of risk of various cancers, including those of the colon,[53][54][55][56][57] [58] lung,[59][60] and possibly the upper GI tract, though some evidence of its effectiveness in preventing cancer of the upper GI tract has been inconclusive.[61][61][62] Its preventative effect against adenocarcinomas may be explained by its inhibition of PTGS2 (COX-2) enzymes expressed in them.[63]
A 2009 article published by the Journal of Clinical Investigation suggested that aspirin might prevent liver damage. In their experiment, scientists from Yale University and The University of Iowa induced damage in certain liver cells (hepatocytes) using excessive doses of acetaminophen. This caused hepatoxicity and hepatocyte death, which triggered an increase in the production of TLR9. The expression of TLR9 caused an inflammatory cascade involving pro–IL-1β and pro-IL-18. Aspirin was found to have a protective effect on hepatocytes because it led to the "downregulation of proinflammatory cytokines".[64]
In another 2009 article published by the Journal of the American Medical Association, men and women who regularly took aspirin after colorectal cancer diagnosis were found to have lower risks of overall and colorectal cancer death compared to patients not using aspirin.[65]
A 2010 article in the Journal of Clinical Oncology has suggested aspirin may reduce the risk of death from breast cancer.[66] While the information has been well-circulated by the media,[67][68] official health bodies and medical groups have expressed concern over the touting of aspirin as a "miracle drug".[69]
A 2010 study by Oxford University involving over 25000 patients showed taking a small (75 mg) daily dose of aspirin for between four and eight years substantially reduces death rates from a range of common cancers by at least a fifth and the reduction of risk continued for 20 years in both men and women. For specific cancers the, reduction was about 40% for bowel cancer, 30% for lung cancer, 10% for prostate cancer and 60% for oesophageal cancer, while the reductions in pancreas, stomach, brain, breast and ovarian cancers were difficult to quantify because there were not enough data, but other studies are in progress. However, taking aspirin doubles the annual risk of major internal bleeding that normally has a very low incidence (about 1 in 1000) in middle age, but increased dramatically after 75 years old.[70]
Resistance
For some people, aspirin does not have as strong an effect on platelets as for others, an effect known as aspirin resistance or insensitivity. One study has suggested women are more likely to be resistant than men,[71] and a different, aggregate study of 2,930 patients found 28% to be resistant.[72] A study in 100 Italian patients found that, of the apparent 31% aspirin-resistant subjects, only 5% were truly resistant, and the others were noncompliant.[73]
Dosage
Adult aspirin tablets are produced in standardised sizes, which vary slightly from country to country, for example 300 mg in Britain and 325 mg in the USA. Smaller doses are based on these standards; thus, 75- and 81-milligram tablets are used; there is no medical significance in the slight difference. It is of historical interest that the US, 325 mg dose is almost exactly equivalent to the historic 5-grain aspirin tablet that preceded the metric dosage.
In general, for adults, doses are taken four times a day for fever or arthritis,[74] with doses near the maximal daily dose used historically for the treatment of rheumatic fever.[75] For the prevention of myocardial infarction in someone with documented or suspected coronary artery disease, much lower doses are taken once daily.[74]
New recommendations from the US Preventive Services Task Force (USPSTF, March, 2009) on the use of aspirin for the primary prevention of coronary heart disease encourage men aged 45–79 and women aged 55–79 to use aspirin when the potential benefit of a reduction in myocardial infarction (MI) for men or stroke for women outweighs the potential harm of an increase in gastrointestinal hemorrhage.[76] The WHI study said regular low dose (75 or 81 mg) aspirin female users had a 25% lower risk of death from cardiovascular disease and a 14% lower risk of death from any cause.[76] Low dose aspirin use was also associated with a trend toward lower risk of cardiovascular events, and lower aspirin doses (75 or 81 mg/day) may optimize efficacy and safety for patients requiring aspirin for long-term prevention.[76]
In children with Kawasaki disease, aspirin is taken at dosages based on body weight, initially four times a day for up to two weeks and then at a lower dose once daily for a further six to eight weeks.[77]
Adverse effects
Contraindications
Aspirin should not be taken by people who are allergic to ibuprofen or naproxen,[78][79] or who have salicylate intolerance[80][81] or a more generalized drug intolerance to NSAIDs, and caution should be exercised in those with asthma or NSAID-precipitated bronchospasm. Owing to its effect on the stomach lining, manufacturers recommend people with peptic ulcers, mild diabetes, or gastritis seek medical advice before using aspirin.[78][82] Even if none of these conditions is present, there is still an increased risk of stomach bleeding when aspirin is taken with alcohol or warfarin.[78][79] Patients with hemophilia or other bleeding tendencies should not take aspirin or other salicylates.[78][82] Aspirin is known to cause hemolytic anemia in people who have the genetic disease glucose-6-phosphate dehydrogenase deficiency (G6PD), in particular in large doses and depending on the severity of the disease.[83] Use of aspirin during dengue fever is not recommended owing to increased bleeding tendency.[84] People with kidney disease, hyperuricemia, or gout should not take aspirin because it inhibits the kidneys' ability to excrete uric acid, and thus may exacerbate these conditions. Aspirin should not be given to children or adolescents to control cold or influenza symptoms, as this has been linked with Reye's syndrome.[6]
Gastrointestinal
Aspirin use has been shown to increase the risk of gastrointestinal bleeding.[85] Although some enteric coated formulations of aspirin are advertised as being "gentle to the stomach", in one study enteric coating did not seem to reduce this risk.[85] Combining aspirin with other NSAIDs has also been shown to further increase this risk.[85] Using aspirin in combination with clopidogrel or warfarin also increases the risk of upper gastrointestinal bleeding.[86]
In addition to enteric coating, "buffering" is the other main method companies have used to try to mitigate the problem of gastrointestinal bleeding. Buffering agents are intended to work by preventing the aspirin from concentrating in the walls of the stomach, although the benefits of buffered aspirin are disputed. Almost any buffering agent used in antacids can be used; Bufferin, for example, uses MgO. Other preparations use CaCO3.[87]
Taking it with vitamin C is a more recently investigated method of protecting the stomach lining. According to research done at a German university, taking equal doses of vitamin C and aspirin decreases the amount of stomach damage that occurs compared to taking aspirin alone.[88][89]
It is reported that deglycyrrhizinated licorice (DGL), an extract of the popular herb licorice, helps relieve the symptoms of gastritis. In a 1979 research study, a dose of 350 milligrams of DGL was shown to decrease the amount of gastrointestinal bleeding induced by three adult-strength aspirin tablets (750 milligrams).[90]
A dose of 500 milligrams of S-adenosyl-methionine (SAMe, an amino acid naturally formed in the body) given together with a large dose of aspirin (1300 milligrams) in a research study reduced the amount of stomach damage by 90 percent.[91]
A study found that, in contrast to oral aspirin, intravenous injection of aspirin did not produce detectable histological damage or significantly alter gastric mucosal potential difference, and concluded that high blood levels of circulating salicylate did not acutely damage gastric mucosa, so that gastric mucosal damage produced acutely after single oral doses of aspirin are due to its topical, rather than systemic, action.[92]
Central effects
Large doses of salicylate, a metabolite of aspirin, have been proposed to cause tinnitus (ringing in the ears) based on experiments in rats, via the action on arachidonic acid and NMDA receptors cascade.[93]
Reye's syndrome
Reye's syndrome, a rare but severe illness characterized by acute encephalopathy and fatty liver, can occur when children or adolescents are given aspirin for a fever or other illnesses or infections. From 1981 through 1997, 1207 cases of Reye's syndrome in under-18 patients were reported to the U.S. Centers for Disease Control and Prevention. Of these, 93% reported being ill in the three weeks preceding onset of Reye's syndrome, most commonly with a respiratory infection, chickenpox, or diarrhea. Salicylates were detectable in 81.9% of children for whom test results were reported.[94] After the association between Reye's syndrome and aspirin was reported and safety measures to prevent it (including a Surgeon General's warning and changes to the labeling of aspirin-containing drugs) were implemented, aspirin taken by children declined considerably in the United States, as did the number of reported cases of Reye's syndrome; a similar decline was found in the United Kingdom after warnings against pediatric aspirin use were issued.[94] The United States Food and Drug Administration now recommends aspirin (or aspirin-containing products) should not be given to anyone under the age of 12 who has a fever,[6] and the British Medicines and Healthcare products Regulatory Agency (MHRA) recommends children who are under 16 years of age should not take aspirin, unless it is on the advice of a doctor.[95]
Hives and swelling
For a small number of people, taking aspirin can result in symptoms that resemble an allergic reaction, including hives, swelling and headache. The reaction is caused by salicylate intolerance and is not a true allergy, but rather an inability to metabolize even small amounts of aspirin, resulting in an overdose.
Other effects
Aspirin can induce angioedema (swelling of skin tissues) in some people. In one study, angioedema appeared one to six hours after ingesting aspirin in some of the patients participating in the study. However, when the aspirin was taken alone, it did not cause angioedema in these patients; the aspirin had been taken in combination with another NSAID-induced drug when angioedema appeared.[96]
Aspirin causes an increased risk of cerebral microbleeds having the appearance on MRI scans of 5–10 mm or smaller hypointense (dark holes) patches.[97][98] Such cerebral microbleeds are important since they often occur prior to ischemic stroke or intracerebral hemorrhage, Binswanger disease and Alzheimer's disease.[original research?]
Aspirin and other NSAIDs can cause hyperkalemia by inducing a hyporenin hypoaldosteronic state via inhibition of prostaglandin synthesis; however, these agents do not typically cause hyperkalemia by themselves in the setting of normal renal function and euvolemic state.[99]
Aspirin can cause prolonged bleeding after operations for up to 10 days. In one study, 30 of 6499 elective surgical patients required reoperations to control bleeding. Twenty had diffuse bleeding and 10 had bleeding from a site. Diffuse, but not discrete, bleeding was associated with the preoperative use of aspirin alone or in combination with other NSAIDS in 19 of the 20 diffuse bleeding patients.[100]
Condition Prothrombin time Partial thromboplastin time Bleeding time Platelet count Vitamin K deficiency or warfarin prolonged normal or mildly prolonged unaffected unaffected Disseminated intravascular coagulation prolonged prolonged prolonged decreased von Willebrand disease unaffected prolonged prolonged unaffected Hemophilia unaffected prolonged unaffected unaffected Aspirin unaffected unaffected prolonged unaffected Thrombocytopenia unaffected unaffected prolonged decreased Liver failure, early prolonged unaffected unaffected unaffected Liver failure, end-stage prolonged prolonged prolonged decreased Uremia unaffected unaffected prolonged unaffected Congenital afibrinogenemia prolonged prolonged prolonged unaffected Factor V deficiency prolonged prolonged unaffected unaffected Factor X deficiency as seen in amyloid purpura prolonged prolonged unaffected unaffected Glanzmann's thrombasthenia unaffected unaffected prolonged unaffected Bernard-Soulier syndrome unaffected unaffected prolonged decreased or unaffected Overdose
Main article: Aspirin poisoningAspirin overdose can be acute or chronic. In acute poisoning, a single large dose is taken; in chronic poisoning, higher than normal doses are taken over a period of time. Acute overdose has a mortality rate of 2%. Chronic overdose is more commonly lethal, with a mortality rate of 25%;[verification needed] chronic overdose may be especially severe in children.[101] Toxicity is managed with a number of potential treatments, including activated charcoal, intravenous dextrose and normal saline, sodium bicarbonate, and dialysis.[102] The diagnosis of poisoning usually involves measurement of plasma salicylate, the active metabolite of aspirin, by automated spectrophotometric methods. Plasma salicylate levels in general range from 30–100 mg/L after usual therapeutic doses, 50–300 mg/L in patients taking high doses and 700–1400 mg/L following acute overdose. Salicylate is also produced as a result of exposure to bismuth subsalicylate, methyl salicylate and sodium salicylate.[103][104]
Interactions
Aspirin is known to interact with other drugs. For example, acetazolamide and ammonium chloride have been known to enhance the intoxicating effect of salicyclates, and alcohol also increases the gastrointestinal bleeding associated with these types of drugs.[78][79] Aspirin is known to displace a number of drugs from protein binding sites in the blood, including the antidiabetic drugs tolbutamide and chlorpropamide, the immunosuppressant methotrexate, phenytoin, probenecid, valproic acid (as well as interfering with beta oxidation, an important part of valproate metabolism) and any nonsteroidal anti-inflammatory drug. Corticosteroids may also reduce the concentration of aspirin. Ibuprofen can negate the antiplatelet effect of aspirin used for cardioprotection and stroke prevention.[105] The pharmacological activity of spironolactone may be reduced by taking aspirin, and aspirin is known to compete with Penicillin G for renal tubular secretion.[106] Aspirin may also inhibit the absorption of vitamin C.[107][108][109]
Chemical properties
Aspirin, an acetyl derivative of salicylic acid, is a white, crystalline, weakly acidic substance, with a melting point of 135 °C (275 °F). Acetylsalicylic acid decomposes rapidly in solutions of ammonium acetate or of the acetates, carbonates, citrates or hydroxides of the alkali metals. Acetylsalicylic acid is stable in dry air, but gradually hydrolyses in contact with moisture to acetic and salicylic acids. In solution with alkalis, the hydrolysis proceeds rapidly and the clear solutions formed may consist entirely of acetate and salicylate.[110]
Synthesis
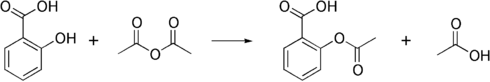
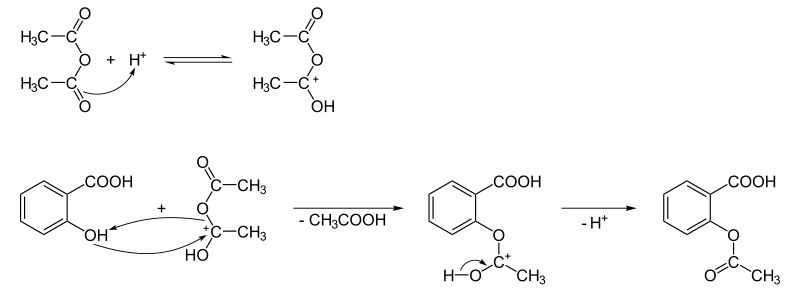
The synthesis of aspirin is classified as an esterification reaction. Salicylic acid is treated with acetic anhydride, an acid derivative, causing a chemical reaction that turns salicylic acid's hydroxyl group into an ester group (R-OH → R-OCOCH3). This process yields aspirin and acetic acid, which is considered a byproduct of this reaction. Small amounts of sulfuric acid (and occasionally phosphoric acid) are almost always used as a catalyst. This method is commonly employed in undergraduate teaching labs.[111]
Formulations containing high concentrations of aspirin often smell like vinegar[112] because aspirin can decompose through hydrolysis in moist conditions, yielding salicylic acid and acetic acid.[113]
The acid dissociation constant (pKa) for acetylsalicylic acid is 3.5 at 25 °C (77 °F).[114]
Polymorphism
Polymorphism, or the ability of a substance to form more than one crystal structure, is important in the development of pharmaceutical ingredients. Many drugs are receiving regulatory approval for only a single crystal form or polymorph. For a long time, only one crystal structure for aspirin was known, although there had been indications aspirin might have a second crystalline form since the 1960s. The elusive second polymorph was first discovered by Vishweshwar and coworkers in 2005,[115] and fine structural details were given by Bond et al.[116] A new crystal type was found after attempted cocrystallization of aspirin and levetiracetam from hot acetonitrile. The form II is only stable at 100 K and reverts to form I at ambient temperature. In the (unambiguous) form I, two salicylic molecules form centrosymmetric dimers through the acetyl groups with the (acidic) methyl proton to carbonyl hydrogen bonds, and in the newly claimed form II, each salicylic molecule forms the same hydrogen bonds with two neighboring molecules instead of one. With respect to the hydrogen bonds formed by the carboxylic acid groups both polymorphs form identical dimer structures.
Mechanism of action
Discovery of the mechanism
In 1971, British pharmacologist John Robert Vane, then employed by the Royal College of Surgeons in London, showed aspirin suppressed the production of prostaglandins and thromboxanes.[117][118] For this discovery, he was awarded both a Nobel Prize in Physiology or Medicine in 1982 and a knighthood.
Suppression of prostaglandins and thromboxanes
Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (PTGS) enzyme required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the PTGS enzyme. This makes aspirin different from other NSAIDs (such as diclofenac and ibuprofen), which are reversible inhibitors.
Low-dose, long-term aspirin use irreversibly blocks the formation of thromboxane A2 in platelets, producing an inhibitory effect on platelet aggregation. This antithrombotic property makes aspirin useful for reducing the incidence of heart attacks.[119] 40 mg of aspirin a day is able to inhibit a large proportion of maximum thromboxane A2 release provoked acutely, with the prostaglandin I2 synthesis being little affected; however, higher doses of aspirin are required to attain further inhibition.[120]
Prostaglandins are local hormones produced in the body and have diverse effects, including the transmission of pain information to the brain, modulation of the hypothalamic thermostat, and inflammation. Thromboxanes are responsible for the aggregation of platelets that form blood clots. Heart attacks are caused primarily by blood clots, and low doses of aspirin are seen as an effective medical intervention for acute myocardial infarction. An unwanted side-effect of the effective anticlotting action of aspirin is that it may cause excessive bleeding.
COX-1 and COX-2 inhibition
There are at least two different types of cyclooxygenase: COX-1 and COX-2. Aspirin irreversibly inhibits COX-1 and modifies the enzymatic activity of COX-2. COX-2 normally produces prostanoids, most of which are proinflammatory. Aspirin-modified PTGS2 produces lipoxins, most of which are anti-inflammatory. Newer NSAID drugs, COX 2 inhibitors, have been developed to inhibit only PTGS2, with the intent to reduce the incidence of gastrointestinal side-effects.[8]
However, several of the new COX 2 inhibitors, such as rofecoxib (Vioxx), have been withdrawn recently, after evidence emerged that PTGS2 inhibitors increase the risk of heart attack. Endothelial cells lining the microvasculature in the body are proposed to express PTGS2, and, by selectively inhibiting PTGS2, prostaglandin production (to be specific, PGI2; prostacyclin) is downregulated with respect to thromboxane levels, as PTGS1 in platelets is unaffected. Thus, the protective anticoagulative effect of PGI2 is removed, increasing the risk of thrombus and associated heart attacks and other circulatory problems. Since platelets have no DNA, they are unable to synthesize new PTGS once aspirin has irreversibly inhibited the enzyme, an important difference with reversible inhibitors.
Additional mechanisms
Aspirin has been shown to have at least three additional modes of action. It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria, by diffusing from the inner membrane space as a proton carrier back into the mitochondrial matrix, where it ionizes once again to release protons.[121] In short, aspirin buffers and transports the protons. When high doses of aspirin are given, it may actually cause fever, owing to the heat released from the electron transport chain, as opposed to the antipyretic action of aspirin seen with lower doses. In addition, aspirin induces the formation of NO-radicals in the body, which have been shown in mice to have an independent mechanism of reducing inflammation. This reduced leukocyte adhesion, which is an important step in immune response to infection; however, there is currently insufficient evidence to show that aspirin helps to fight infection.[122] More recent data also suggests that salicylic acid and its derivatives modulate signaling through NF-κB.[123] NF-κB, a transcription factor complex, plays a central role in many biological processes, including inflammation.
Effects upon hypothalamic-pituitary-adrenal activity
Aspirin, like other medications affecting prostaglandin synthesis, has profound effects on the pituitary gland, which indirectly affects a number of other hormones and physiological functions. Effects on growth hormone, prolactin,[124] and TSH (with relevant effect on T3 and T4) were observed directly.[125] Aspirin reduces the effects of vasopressin[126] and increases those of naloxone[127] upon the secretion of ACTH and cortisol by the hypothalamic-pituitary-adrenal axis (HPA axis), which has been suggested to occur through an interaction with endogenous prostaglandins and their role in regulating the HPA axis.[126]
Pharmacokinetics
Salicylic acid is a weak acid, and very little of it is ionized in the stomach after oral administration. Acetylsalicylic acid is poorly soluble in the acidic conditions of the stomach, which can delay absorption of high doses for eight to 24 hours. The increased pH and larger surface area of the small intestine causes aspirin to be absorbed rapidly there, which in turn allows more of the salicylate to dissolve. Owing to the issue of solubility, however, aspirin is absorbed much more slowly during overdose, and plasma concentrations can continue to rise for up to 24 hours after ingestion.[128][129][130]
About 50–80% of salicylate in the blood is bound by protein, while the rest remains in the active, ionized state; protein binding is concentration-dependent. Saturation of binding sites leads to more free salicylate and increased toxicity. The volume of distribution is 0.1–0.2 l/kg. Acidosis increases the volume of distribution because of enhancement of tissue penetration of salicylates.[130]
As much as 80% of therapeutic doses of salicylic acid is metabolized in the liver. Conjugation with glycine forms salicyluric acid, and with glucuronic acid it forms salicyl acyl and phenolic glucuronide. These metabolic pathways have only a limited capacity. Small amounts of salicylic acid are also hydroxylated to gentisic acid. With large salicylate doses, the kinetics switch from first order to zero order, as metabolic pathways become saturated and renal excretion becomes increasingly important.[130]
Salicylates are excreted mainly by the kidneys as salicyluric acid (75%), free salicylic acid (10%), salicylic phenol (10%), and acyl glucuronides (5%), gentisic acid (< 1%), and 2,3-dihydroxybenzoic acid.[131] When small doses (less than 250 mg in an adult) are ingested, all pathways proceed by first-order kinetics, with an elimination half-life of about 2.0 to 4.5 hours.[132][133] When higher doses of salicylate are ingested (more than 4 g), the half-life becomes much longer (15–30 hours),[134] because the biotransformation pathways concerned with the formation of salicyluric acid and salicyl phenolic glucuronide become saturated.[135] Renal excretion of salicylic acid becomes increasingly important as the metabolic pathways become saturated, because it is extremely sensitive to changes in urinary pH. There is a 10- to 20-fold increase in renal clearance when urine pH is increased from 5 to 8. The use of urinary alkalinization exploits this particular aspect of salicylate elimination.[136]
History
Plant extracts, including willow bark and spiraea, of which salicylic acid was the active ingredient, had been known to help alleviate headaches, pains, and fevers since antiquity. The father of modern medicine, Hippocrates, who lived sometime between 460 BC and 377 BC, left historical records describing the use of powder made from the bark and leaves of the willow tree to help these symptoms.[137]
A French chemist, Charles Frederic Gerhardt, was the first to prepare acetylsalicylic acid in 1853. In the course of his work on the synthesis and properties of various acid anhydrides, he mixed acetyl chloride with a sodium salt of salicylic acid (sodium salicylate). A vigorous reaction ensued, and the resulting melt soon solidified.[138] Since no structural theory existed at that time, Gerhardt called the compound he obtained "salicylic-acetic anhydride" (wasserfreie Salicylsäure-Essigsäure). This preparation of aspirin ("salicylic-acetic anhydride") was one of the many reactions Gerhardt conducted for his paper on anhydrides and he did not pursue it further.
Six years later, in 1859, von Gilm obtained analytically pure acetylsalicylic acid (which he called acetylierte Salicylsäure, acetylated salicylic acid) by a reaction of salicylic acid and acetyl chloride.[139] In 1869, Schröder, Prinzhorn and Kraut repeated both Gerhardt's (from sodium salicylate) and von Gilm's (from salicylic acid) syntheses and concluded both reactions gave the same compound—acetylsalicylic acid. They were first to assign to it the correct structure with the acetyl group connected to the phenolic oxygen.[140]
In 1897, chemists working at Bayer AG produced a synthetically altered version of salicin, derived from the species meadowsweet (filipendula ulmaria), which caused less digestive upset than pure salicylic acid. The identity of the lead chemist on this project is a matter of controversy. Bayer's states that the work was done by Felix Hoffmann, but the Jewish chemist Arthur Eichengrün later claimed he was the lead investigator and records of his contribution were expunged under the Nazi regime.[141][142] The new drug, formally acetylsalicylic acid, was named Aspirin by Bayer AG after the old botanical name for meadowsweet, Spiraea ulmaria. By 1899, Bayer was selling it around the world.[143] The name Aspirin is derived from acetyl and spirsäure, an old German name for salicylic acid.[144] The popularity of aspirin grew over the first half of the 20th century, spurred by its supposed effectiveness in the wake of the Spanish flu pandemic of 1918. However, recent research suggests the high death toll of the 1918 flu was partly due to aspirin, as the doses used at times can lead to toxicity, fluid in the lungs, and, in some cases, contribute to secondary bacterial infections and mortality.[145] Aspirin's profitability led to fierce competition and the proliferation of aspirin brands and products, especially after the American patent held by Bayer expired in 1917.[146][147]
The popularity of aspirin declined after the market releases of paracetamol (acetaminophen) in 1956 and ibuprofen in 1969.[148] In the 1960s and 1970s, John Vane and others discovered the basic mechanism of aspirin's effects, while clinical trials and other studies from the 1960s to the 1980s established aspirin's efficacy as an anticlotting agent that reduces the risk of clotting diseases.[149] Aspirin sales revived considerably in the last decades of the 20th century, and remain strong in the 21st century, because of its widespread use as a preventive treatment for heart attacks and strokes.[150]
Trademark
As part of war reparations specified in the 1919 Treaty of Versailles following Germany's surrender after World War I, Aspirin (along with heroin) lost its status as a registered trademark in France, Russia, the United Kingdom, and the United States, where it became a generic name.[151][152][153] Today, "aspirin" is a generic word in Australia, France, India, Ireland, New Zealand, Pakistan, Jamaica, the Philippines, South Africa, United Kingdom and the United States.[154] Aspirin, with a capital "A", remains a registered trademark of Bayer in Germany, Canada, Mexico, and in over 80 other countries, where the trademark is owned by Bayer, using acetylsalicylic acid in all markets, but using different packaging and physical aspects for each.[155][156]
Compendial status
Veterinary use
Aspirin is sometimes used for pain relief or as an anti-coagulant in veterinary medicine, primarily in dogs and sometimes horses, although newer medications with fewer side-effects are generally used instead. Both dogs and horses are susceptible to the gastrointestinal side-effects associated with salicylates, but it is a convenient treatment for arthritis in older dogs and has shown some promise in cases of laminitis in horses.[159][160] Aspirin should be used in animals only under the direct supervision of a veterinarian; in particular, cats lack the glucuronide conjugates that aid in the excretion of aspirin, making even low doses potentially toxic.[161]
See also
- Aspirin poisoning
- History of aspirin
- NSAID
References
- ^ http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1119266/?tool=pmcentrez
- ^ Paterson, John R.; Baxter, Gwendoline; Dreyer, Jacob S.; Halket, John M.; Flynn, Robert; Lawrence, James R. (2008). "Salicylic Acid sans Aspirin in Animals and Man: Persistence in Fasting and Biosynthesis from Benzoic Acid". Journal of Agricultural and Food Chemistry 56 (24): 11648–11652. doi:10.1021/jf800974z. PMC 2800778. PMID 19053387. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2800778.
- ^ Lewis, H D; J W Davis, D G Archibald, W E Steinke, T C Smitherman, J E Doherty, H W Schnaper, M M LeWinter, E Linares, J M Pouget, S C Sabharwal, E Chesler, H DeMots (1983-08-18). "Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study". The New England journal of medicine 309 (7): 396–403. doi:10.1056/NEJM198308183090703. ISSN 00284793. PMID 6135989.
- ^ Julian, D G; D A Chamberlain, S J Pocock (1996-09-24). "A comparison of aspirin and anticoagulation following thrombolysis for myocardial infarction (the AFTER study): a multicentre unblinded randomised clinical trial". BMJ (British Medical Journal) 313 (7070): 1429–1431. PMC 2353012. PMID 8973228. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2353012.
- ^ Krumholz, Harlan M.; Martha J. Radford, Edward F. Ellerbeck, John Hennen, Thomas P. Meehan, Marcia Petrillo, Yun Wang, Timothy F. Kresowik, Stephen F. Jencks (1995). "Aspirin in the Treatment of Acute Myocardial Infarction in Elderly Medicare Beneficiaries : Patterns of Use and Outcomes". Circulation 92 (10): 2841–2847. PMID 7586250.
- ^ a b c Macdonald S (2002). "Aspirin use to be banned in under 16 year olds". BMJ 325 (7371): 988. doi:10.1136/bmj.325.7371.988/c. PMC 1169585. PMID 12411346. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1169585.
- ^ Burke, Anne; Smyth, Emer; FitzGerald, Garret A. (2006). Chapter 26: Analgesic Antipyretic and Antiinflammatory Agents in Goodman and Gilman's the pharmacological basis of therpeutics.. New York: McGraw-Hill. pp. 671–716. ISBN 0071422803. http://www.accessmedicine.com/content.aspx?aID=942410&searchStr=aspirin.
- ^ a b Warner, T. D.; Warner TD, Mitchell JA. (2002). "Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum?". Proc Natl Acad Sci USA 99 (21): 13371–3. doi:10.1073/pnas.222543099. PMC 129677. PMID 12374850. http://www.pnas.org/cgi/content/extract/99/21/13371.
- ^ "The use of aspirin". Wordconstructions.com. http://www.wordconstructions.com/articles/health/aspirin.html. Retrieved 2011-05-11.
- ^ a b "Aspirin". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/aspirin.html. Retrieved 3 April 2011.
- ^ Gaciong (2003). "The real dimension of analgesic activity of aspirin". Thrombosis research 110 (5–6): 361–364. doi:10.1016/j.thromres.2003.08.009. PMID 14592563.
- ^ a b Hersh, E.; Moore, P.; Ross, G. (2000). "Over-the-counter analgesics and antipyretics: A critical assessment". Clinical Therapeutics 22 (5): 500–548. doi:10.1016/S0149-2918(00)80043-0. PMID 10868553.
- ^ Zhang; Po, A. L. (1997). "Do codeine and caffeine enhance the analgesic effect of aspirin?--A systematic overview". Journal of clinical pharmacy and therapeutics 22 (2): 79–97. doi:10.1111/j.1365-2710.1997.tb00002.x. PMID 9373807.
- ^ Cooper; Engel, J.; Ladov, M.; Precheur, H.; Rosenheck, A.; Rauch, D. (1982). "Analgesic efficacy of an ibuprofen-codeine combination". Pharmacotherapy 2 (3): 162–167. PMID 6763202.
- ^ Zhang; Li Wan Po, A. (1998). "Efficacy of minor analgesics in primary dysmenorrhoea: a systematic review". British journal of obstetrics and gynaecology 105 (7): 780–789. doi:10.1111/j.1471-0528.1998.tb10210.x. PMID 9692420.
- ^ Cook; O'Connor, P. J.; Eubanks, S. A.; Smith, J. C.; Lee, M. (1997). "Naturally occurring muscle pain during exercise: assessment and experimental evidence". Medicine and science in sports and exercise 29 (8): 999–1012. doi:10.1097/00005768-199708000-00004. PMID 9268956.
- ^ Gliottoni; Motl, R. W. (2008). "Effect of caffeine on leg-muscle pain during intense cycling exercise: possible role of anxiety sensitivity". International journal of sport nutrition and exercise metabolism 18 (2): 103–115. PMID 18458355.
- ^ Motl; O'Connor, P. J.; Dishman, R. K. (2003). "Effect of caffeine on perceptions of leg muscle pain during moderate intensity cycling exercise". The journal of pain : official journal of the American Pain Society 4 (6): 316–321. doi:10.1016/S1526-5900(03)00635-7. PMID 14622688.
- ^ Barlas, P.; Craig, J.; Robinson, J.; Walsh, D.; Baxter, G.; Allen, J. (2000). "Managing delayed-onset muscle soreness: Lack of effect of selected oral systemic analgesics". Archives of Physical Medicine and Rehabilitation 81 (7): 966–972. doi:10.1053/apmr.2000.6277. PMID 10896014.
- ^ Tfelt-Hansen P (2008). "Triptans vs Other Drugs for Acute Migraine. Are There Differences in Efficacy? A Comment". Headache 48 (4): 601–605. doi:10.1111/j.1526-4610.2008.01064.x. PMID 18377382.
- ^ Lampl C, Voelker M, Diener HC (2007). "Efficacy and safety of 1,000 mg effervescent aspirin: individual patient data meta-analysis of three trials in migraine headache and migraine accompanying symptoms". J Neurol 254 (6): 705–712. doi:10.1007/s00415-007-0547-2. PMID 17406776.
- ^ a b Diener HC, Bussone G, de Liano H et al (2004). "The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study". Cephalalgia 25 (10): 776–787. doi:10.1111/j.1468-2982.2005.00948.x. PMID 16162254.
- ^ Diener HC, Pfaffenrath V, Pageler L et al (2004). "Efficacy and safety of 1,000 mg effervescent aspirin: individual patient data meta-analysis of three trials in migraine headache and migraine accompanying symptoms". Cephalalgia 24 (11): 947–54. doi:10.1111/j.1468-2982.2004.00783.x. PMID 15482357.
- ^ Goldstein J, Silberstein SD, Saper JR et al (2006). "Acetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double-blind, randomized, parallel-group, single-dose, placebo-controlled study". Headache 46 (3): 444–53. doi:10.1111/j.1526-4610.2006.00376.x. PMID 16618262.
- ^ Goldstein J, Silberstein SD, Saper JR et al (2005). "Acetaminophen, aspirin, and caffeine versus sumatriptan succinate in the early treatment of migraine: results from the ASSET trial". Headache 45 (8): 973–82. doi:10.1111/j.1526-4610.2005.05177.x. PMID 16109110.
- ^ a b Martínez-Martín, P.; Raffaelli E, E.; Titus, F.; Despuig, J.; Fragoso, Y. D.; Díez-Tejedor, E.; Liaño, H.; Leira, R. et al. (2001). "Efficacy and safety of metamizol vs. Acetylsalicylic acid in patients with moderate episodic tension-type headache: a randomized, double-blind, placebo- and active-controlled, multicentre study". Cephalalgia : an international journal of headache 21 (5): 604–610. doi:10.1046/j.1468-2982.2001.00216.x. PMID 11472387.
- ^ a b Steiner, T. J.; Lange, R.; Voelker, M. (2003). "Aspirin in episodic tension-type headache: placebo-controlled dose-ranging comparison with paracetamol". Cephalalgia : an international journal of headache 23 (1): 59–66. doi:10.1046/j.1468-2982.2003.00470.x. PMID 12534583.
- ^ Nebe, J.; Heier, M.; Diener, H. C. (1995). "Low-dose ibuprofen in self-medication of mild to moderate headache: a comparison with acetylsalicylic acid and placebo". Cephalalgia : an international journal of headache 15 (6): 531–535. doi:10.1046/j.1468-2982.1995.1506531.x. PMID 8706118.
- ^ Migliardi, J. R.; Armellino; Friedman; Gillings; Beaver (1994). "Caffeine as an analgesic adjuvant in tension headache". Clinical pharmacology and therapeutics 56 (5): 576–586. doi:10.1038/clpt.1994.179. PMID 7955822.
- ^ "Aspirin and Migraine". National Headache Foundation. http://www.headaches.org/education/Medications/Aspirin_and_Migraine. Retrieved 2011-03-28.
- ^ K.A. Fackelmann (1990-02-17). "Low dose of aspirin keeps migraine away". Science News. http://findarticles.com/p/articles/mi_m1200/is_n7_v137/ai_8556715/. Retrieved 2011-03-28.
- ^ Dalessio. D.J. (1990-02-17). "Aspirin prophylaxis for migraine". ISSN 0098-7484. http://www.faqs.org/abstracts/Health/Aspirin-prophylaxis-for-migraine-A-meta-analysis-of-low-dose-aspirin-for-the-prevention-of-pregnancy.html. Retrieved 2011-03-28.
- ^ Hennekens, Charles H., Buring, Julie E., Peto, Richard (1990). "Low-dose aspirin for migraine prophylaxis". JAMA, The Journal of the American Medical Association. ISSN 0098-7484. http://www.ncbi.nlm.nih.gov/pubmed/2204739. Retrieved 2011-03-28.
- ^ a b c d e Baigent C, Blackwell L, Collins R, et al. (2009). "Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials". Lancet 373 (9678): 1849–60. doi:10.1016/S0140-6736(09)60503-1. PMC 2715005. PMID 19482214. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2715005.
- ^ Wolff T, Miller T, Ko S (2009). "Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force". Ann. Intern. Med. 150 (6): 405–10. PMID 19293073.
- ^ US Preventive Services Task Force (2009). "Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement". Ann. Intern. Med. 150 (6): 396–404. PMID 19293072.
- ^ a b Bachert, C.; Chuchalin, A.; Eisebitt, R.; Netayzhenko, V.; Voelker, M. (2005). "Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study". Clinical therapeutics 27 (7): 993–1003. doi:10.1016/j.clinthera.2005.06.002. PMID 16154478.
- ^ Eccles, R.; Loose, I.; Jawad, M.; Nyman, L. (2003). "Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection". Pain medicine (Malden, Mass.) 4 (2): 118–124. doi:10.1046/j.1526-4637.2003.03019.x. PMID 12873261.
- ^ a b c National Heart Foundation of Australia (RF/RHD guideline development working group) and the Cardiac Society of Australia and New Zealand (2006). "Diagnosis and management of acute rheumatic fever and rheumatic heart disease in Australia. An evidence-based review" (PDF). National Heart Foundation of Australia. pp. 33–37. http://www.racgp.org.au/Content/NavigationMenu/ClinicalResources/RACGPGuidelines/DiagnosisandmanagementofacuterheumaticfeverandrheumaticheartdiseaseinAustralia/NHFA-CSANZ_ARF_RHD_2006.pdf. Retrieved 2011-05-09.
- ^ Karademir; Oğuz, D.; Senocak, F.; Ocal, B.; Karakurt, C.; Cabuk, F. (2003). "Tolmetin and salicylate therapy in acute rheumatic fever: Comparison of clinical efficacy and side-effects". Pediatrics international : official journal of the Japan Pediatric Society 45 (6): 676–679. PMID 14651540.
- ^ Singh; Chugh, J. C.; Shembesh, A. H.; Ben-Musa, A. A.; Mehta, H. C. (1992). "Hepatotoxicity of high dose salicylate therapy in acute rheumatic fever". Annals of tropical paediatrics 12 (1): 37–40. PMID 1376585.
- ^ Hashkes; Tauber, T.; Somekh, E.; Brik, R.; Barash, J.; Mukamel, M.; Harel, L.; Lorber, A. et al. (2003). "Naproxen as an alternative to aspirin for the treatment of arthritis of rheumatic fever: a randomized trial". The Journal of pediatrics 143 (3): 399–401. doi:10.1067/S0022-3476(03)00388-3. PMID 14517527.
- ^ Hsieh KS, Weng KP, Lin CC, Huang TC, Lee CL, Huang SM (2004). "Treatment of acute Kawasaki disease: aspirin's role in the febrile stage revisited". Pediatrics 114 (6): e689–93. doi:10.1542/peds.2004-1037. PMID 15545617. http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=15545617.
- ^ Krumholz, HM; Radford MJ, Ellerbeck EF, Hennen J, Meehan TP, Petrillo M, Wang Y, Kresowik TF, Jencks SF. (1995). "Aspirin in the treatment of acute myocardial infarction in elderly Medicare beneficiaries. Patterns of use and outcomes". Circulation 92 (10): 2841–7. PMID 7586250.
- ^ ISIS-2 Collaborative group (1988). "Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2". Lancet 2 (2): 349–60. PMID 2899772.
- ^ Mallinson, T (2010). "Myocardial Infarction". Focus on First Aid (15): 15. http://www.focusonfirstaid.co.uk/Magazine/issue15/index.aspx. Retrieved 2010-06-08.
- ^ "Loke and Dery: Air Travel and Venous Thrombosis: How Much Help Might Aspirin Be? 2002". Medscape.com. http://www.medscape.com/viewarticle/441153. Retrieved 2011-05-11.
- ^ Chew EY, Williams GA, Burton TC, Barton FB, Remaley NA, Ferris FL (1992). "Aspirin effects on the development of cataracts in patients with diabetes mellitus. Early treatment diabetic retinopathy study report 16". Arch Ophthalmol 110 (3): 339–42. PMID 1543449.
- ^ Bosetti, et al.; Talamini, R; Negri, E; Franceschi, S; Montella, M; La Vecchia, C (2006). "Aspirin and the risk of prostate cancer". Eur J Cancer Prev 15 (1): 43–5. doi:10.1097/01.cej.0000180665.04335.de. PMID 16374228.
- ^ Menezes, et al.; Swede, H; Niles, R; Moysich, KB (2006). "Regular use of aspirin and prostate cancer risk (United States)". Cancer Causes & Control 17 (3): 251–6. doi:10.1007/s10552-005-0450-z. PMID 16489532.
- ^ Schernhammer, et al.; Kang, JH; Chan, AT; Michaud, DS; Skinner, HG; Giovannucci, E; Colditz, GA; Fuchs, CS (2004). "A Prospective Study of Aspirin Use and the Risk of Pancreatic Cancer in Women". J Natl Cancer Inst 96 (1): 22–28. doi:10.1093/jnci/djh001. PMID 14709735. http://jnci.oxfordjournals.org/cgi/content/full/96/1/22.
- ^ Larsson SC, Giovannucci E, Bergkvist L, Wolk A (2006). "Aspirin and nonsteroidal anti-inflammatory drug use and risk of pancreatic cancer: a meta-analysis". Cancer Epidemiol. Biomarkers Prev. 15 (12): 2561–4. doi:10.1158/1055-9965.EPI-06-0574. PMID 17164387. http://cebp.aacrjournals.org/cgi/content/full/15/12/2561.
- ^ Thun MJ, Namboodiri MM, Heath CW (1991). "Aspirin use and reduced risk of fatal colon cancer". N Engl J Med 325 (23): 1593–6. doi:10.1056/NEJM199112053252301. PMID 1669840.
- ^ Baron, et al.; Cole, BF; Sandler, RS; Haile, RW; Ahnen, D; Bresalier, R; McKeown-Eyssen, G; Summers, RW et al. (2003). "A randomized trial of aspirin to prevent colorectal adenomas". N Engl J Med 348 (10): 891–9. doi:10.1056/NEJMoa021735. PMID 12621133.
- ^ Chan, et al.; Giovannucci, EL; Schernhammer, ES; Colditz, GA; Hunter, DJ; Willett, WC; Fuchs, CS (2004). "A Prospective Study of Aspirin Use and the Risk for Colorectal Adenoma". Ann Intern Med 140 (3): 157–66. PMID 14757613.
- ^ Chan, et al.; Giovannucci, EL; Meyerhardt, JA; Schernhammer, ES; Curhan, GC; Fuchs, CS (2005). "Long-term Use of Aspirin and Nonsteroidal Anti-inflammatory Drugs and Risk of Colorectal Cancer". JAMA 294 (8): 914–23. doi:10.1001/jama.294.8.914. PMC 1550973. PMID 16118381. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1550973.
- ^ Prof Peter M Rothwell FMedSci, Michelle Wilson BSc, Carl-Eric Elwin MD, Prof Bo Norrving PhD, Prof Ale Algra MD, Prof Charles P Warlow FMedSci, Prof Tom W Meade FRS (2010). "Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials". The Lancet 376 (9754): 1741–1750. doi:10.1016/S0140-6736(10)61543-7.
- ^ "Aspirin may help fight colon cancer - USATODAY.com". Yourlife.usatoday.com. http://yourlife.usatoday.com/health/story/2011-10-27/Aspirin-reduces-colon-cancer-risk-by-60/50963272/1. Retrieved 2011-11-09.
- ^ Akhmedkhanov, et al.; Toniolo, P; Zeleniuch-Jacquotte, A; Koenig, KL; Shore, RE (2002). "Aspirin and lung cancer in women". Br J cancer 87 (11): 1337–8. doi:10.1038/sj.bjc.6600370. PMC 2364276. PMID 12085255. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2364276.
- ^ Moysich KB, Menezes RJ, Ronsani A, et al. (2002). "Regular aspirin use and lung cancer risk". BMC Cancer 2: 31. doi:10.1186/1471-2407-2-31. PMC 138809. PMID 12453317. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=138809. Free full text
- ^ a b Jayaprakash, Vijayvel; Jayaprakash V, Menezes RJ, Javle MM, McCann SE, Baker JA, Reid ME, Natarajan N, Moysich KB. (2006). "Regular aspirin use and esophageal cancer risk". Int J Cancer 119 (1): 202–7. doi:10.1002/ijc.21814. PMID 16450404.
- ^ Bosetti, et al.; Talamini, R; Franceschi, S; Negri, E; Garavello, W; La Vecchia, C (2003). "Aspirin use and cancers of the upper aerodigestive tract". Br J Cancer 88 (5): 672–74. doi:10.1038/sj.bjc.6600820. PMC 2376339. PMID 12618872. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2376339.
- ^ Wolff, et al.; Saukkonen, K; Anttila, S; Karjalainen, A; Vainio, H; Ristimäki, A (15 November 1998). "Expression of cyclooxygenase-2 in human lung carcinoma". Cancer Research 58 (22): 4997–5001. PMID 9823297. http://cancerres.aacrjournals.org/cgi/content/abstract/58/22/4997.
- ^ Imaeda, Avlin B.; Watanabe, Azuma; Sohail, Muhammad A.; Mahmood, Shamail; Mohamadnejad, Mehdi; Sutterwala, Fayyaz S.; Flavell, Richard A.; Mehal, Wajahat Z. (2009). "Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome". Journal of Clinical Investigation 119 (2): 305–14. doi:10.1172/JCI35958. PMC 2631294. PMID 19164858. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2631294.
- ^ Chan, et al.; Ogino, S; Fuchs, CS (12 August 209). "Aspirin Use and Survival After Diagnosis of Colorectal Cancer". JAMA 302 (6): 649–658. doi:10.1001/jama.2009.1112. PMC 2848289. PMID 19671906. http://jama.ama-assn.org/cgi/content/short/302/6/649?rss=1.
- ^ Holmes, M.; et al. (2010). "Aspirin intake and survival after breast cancer". Journal of Clinical Oncology 28 (9): 1467–1472. doi:10.1200/JCO.2009.22.7918. PMC 2849768. PMID 20159825. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2849768.
- ^ "Is aspirin a miracle drug?". ABC News. 2010. http://abcnews.go.com/Health/video/aspirin-miracle-drug-9980248.
- ^ Coomer, C. (2010). "Aspirin battling breast cancer". Fox News Health Blog. http://health.blogs.foxnews.com/2010/02/17/aspirin-battling-breast-cancer/.
- ^ "Women warned aspirin reports may be misleading". National Prescribing Service. 2010. http://www.nps.org.au/news_and_media/media_releases/repository/Women_warned_aspirin.
- ^ "Small daily aspirin dose 'cuts cancer risk". BBC News Health (2010), available at http://www.bbc.co.uk/news/health-11930988
- ^ Dorsch MP, Lee JS, Lynch DR, Dunn SP, Rodgers JE, Schwartz T, Colby E, Montague D, Smyth SS (2007). "Aspirin Resistance in Patients with Stable Coronary Artery Disease with and without a History of Myocardial Infarction". Ann Pharmacother 41 (May): 737. doi:10.1345/aph.1H621. PMID 17456544.
- ^ Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR (2008). "Aspirin "resistance" and risk of cardiovascular morbidity: systematic review and meta-analysis". BMJ 336 (7637): 195–8. doi:10.1136/bmj.39430.529549.BE. PMC 2213873. PMID 18202034. http://bmj.com/cgi/pmidlookup?view=long&pmid=18202034.
- ^ Pignatelli P, Di Santo S, Barillà F, Gaudio C, Violi F (2008). "Multiple anti-atherosclerotic treatments impair aspirin compliance: effects on aspirin resistance". J. Thromb. Haemost. 6 (10): 1832–4. doi:10.1111/j.1538-7836.2008.03122.x. PMID 18680540.
- ^ a b British National Formulary (45 ed.). British Medical Journal and Royal Pharmaceutical Society of Great Britain. 2003.
- ^ "Aspirin monograph: dosages, etc". Medscape.com. http://www.medscape.com/druginfo/monograph?cid=med&drugid=3881&drugname=Aspirin+EC+Oral&monotype=monograph. Retrieved 2011-05-11.
- ^ a b c "Medscape: Medscape Access". Cme.medscape.com. http://cme.medscape.com/viewarticle/589895?src=cmemp. Retrieved 2011-05-11.
- ^ British National Formulary for Children. British Medical Journal and Royal Pharmaceutical Society. 2006.
- ^ a b c d e "Aspirin information from Drugs.com". Drugs.com. http://www.drugs.com/aspirin.html. Retrieved 2008-05-08.
- ^ a b c "Oral Aspirin information". First DataBank. http://www.personalmd.com/drgdb/3.htm. Retrieved 2008-05-08.
- ^ Raithel M, Baenkler HW, Naegel A, et al. (September 2005). "Significance of salicylate intolerance in diseases of the lower gastrointestinal tract" (PDF). J. Physiol. Pharmacol. 56 Suppl 5: 89–102. PMID 16247191. http://www.jpp.krakow.pl/journal/archive/09_05_s5/pdf/89_09_05_s5_article.pdf.
- ^ Senna GE, Andri G, Dama AR, Mezzelani P, Andri L (1995). "Tolerability of imidazole salycilate in aspirin-sensitive patients". Allergy Proc 16 (5): 251–4. doi:10.2500/108854195778702675. PMID 8566739. http://openurl.ingenta.com/content/nlm?genre=article&issn=1088-5412&volume=16&issue=5&spage=251&aulast=Senna.
- ^ a b "PDR Guide to Over the Counter (OTC) Drugs". http://www.mercksource.com/pp/us/cns/cns_hl_pdr.jspzQzpgzEzzSzppdocszSzuszSzcnszSzcontentzSzpdrotczSzotc_fullzSzdrugszSzfgotc036zPzhtm. Retrieved 2008-04-28.
- ^ Frank B. Livingstone. (1985). Frequencies of hemoglobin variants: thalassemia, the glucose-6-phosphate dehydrogenase deficiency, G6PD variants, and ovalocytosis in human populations. Oxford University Press. ISBN 0195036344. http://books.google.com/?id=OjqNeJERhWwC&q=0195036344&dq=0195036344. Retrieved 2011-05-07.
- ^ "Dengue and Dengue Hemorrhagic Fever: Information for Health Care Practitioners". http://www.cdc.gov/NCIDOD/dvbid/dengue/dengue-hcp.htm. Retrieved 2008-04-28.
- ^ a b c Sørensen HT, Mellemkjaer L, Blot WJ, et al. (2000). "Risk of upper gastrointestinal bleeding associated with use of low-dose aspirin". Am. J. Gastroenterol. 95 (9): 2218–24. doi:10.1111/j.1572-0241.2000.02248.x. PMID 11007221. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0002-9270&date=2000&volume=95&issue=9&spage=2218.
- ^ Delaney JA, Opatrny L, Brophy JM & Suissa S (2007). "Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding". CMAJ 177 (4): 347–51. doi:10.1503/cmaj.070186. PMC 1942107. PMID 17698822. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1942107.
- ^ "General Chemistry Online: FAQ: Acids and bases: What is the buffer system in buffered aspirin?". Antoine.frostburg.edu. http://antoine.frostburg.edu/chem/senese/101/acidbase/faq/buffered-aspirin.shtml. Retrieved 2011-05-11.
- ^ Dammannet al. (2004). "Effects of buffered and plain acetylsalicylic acid formulations with and without ascorbic acid on gastric mucosa in healthy subjects". Aliment Pharmacol Ther. (19): 367–74.
- ^ Kontureket al.; Kania, J; Hahn, EG; Konturek, JW (2006). "Ascorbic acid attenuates aspirin-induced gastric damage: role of inducible nitric oxide synthase". J Physiol Pharmacol. 57 Suppl 5 (5): 125–36. PMID 17218764. http://www.ncbi.nlm.nih.gov/pubmed/17218764.
- ^ Reeseet al. (1979). "Effect of deglycyrrhinized liquorice on gastric mucosal damage by aspirin". Scand J Gastroenterol (14): 605–07.
- ^ Laudanno et al. (1984). "Prostaglandin E1 (misoprostol) and S-adenosylmethionine in the prevention of hemorrhagic gastritis induced by aspirin in the human. Endoscopic, histologic and histochemical study". Acta Gastroenterol Latinoam (14): 289–93.
- ^ Kevin J. Ivey, Douglas B. Paone and William J. Krause, Digestive Diseases and Sciences 25, 97-99, doi:10.1007/BF01308304, Acute effect of systemic aspirin on gastric mucosa in man
- ^ Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL (2003). "Salicylate induces tinnitus through activation of cochlear NMDA receptors". J. Neurosci. 23 (9): 3944–52. PMID 12736364. http://www.jneurosci.org/cgi/content/full/23/9/3944.
- ^ a b Belay ED, Bresee JS, Holman RC, Khan AS, Shahriari A, Schonberger LB (1999). "Reye's syndrome in the United States from 1981 through 1997". N. Engl. J. Med. 340 (18): 1377–82. doi:10.1056/NEJM199905063401801. PMID 10228187. http://content.nejm.org/cgi/pmidlookup?view=short&pmid=10228187&promo=ONFLNS19.
- ^ NHS Choices: Reye's syndrome. Last reviewed: 16 December 2008 http://www.nhs.uk/conditions/Reyes-syndrome/Pages/Introduction.aspx
- ^ Berges-Gimeno MP & Stevenson DD (2004). "Nonsteroidal anti-inflammatory drug-induced reactions and desensitization". J Asthma 41 (4): 375–84. doi:10.1081/JAS-120037650. PMID 15281324.
- ^ Vernooij MW, Haag MD, der Lugt A, Hofman A, Krestin GP, Stricker BH, Breteler MM (2009). "Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study". Arch Neurol 66 (6): 714–20. doi:10.1001/archneurol.2009.42. PMID 19364926.
- ^ Gorelick PB (2009). "Cerebral microbleeds: evidence of heightened risk associated with aspirin use". Arch Neurol 66 (6): 691–3. doi:10.1001/archneurol.2009.85. PMID 19506128.
- ^ Medical knowledge self-assessment program for students 4, By American College of Physicians, Clerkship Directors in Internal Medicine, Nephrology 227, Item 29
- ^ Scher, K.S. (1996). "Unplanned reoperation for bleeding". Am Surg 62 (1): 52–55. PMID 8540646.
- ^ Gaudreault P, Temple AR, Lovejoy FH Jr (1982). "The relative severity of acute versus chronic salicylate poisoning in children: a clinical comparison". Pediatrics 70 (4): 566–9. PMID 7122154. (primary source)[unreliable medical source?]
- ^ Marx, John (2006). Rosen's emergency medicine: concepts and clinical practice. Mosby/Elsevier. p. 2242. ISBN 9780323028455.
- ^ Morra P, Bartle WR, Walker SE, Lee SN, Bowles SK, Reeves RA (1996). "Serum concentrations of salicylic acid following topically applied salicylate derivatives". Ann. Pharmacother 30 (9): 935–40. PMID 8876850.
- ^ R. Baselt (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, California: Biomedical Publications. pp. 22–5.
- ^ "Information for Healthcare Professionals: Concomitant Use of Ibuprofen and Aspirin". FDA. U.S. Department of Health & Human Services. 2006-09. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm125222.htm. Retrieved 2010-11-22.
- ^ Katzung (1998), p. 584.
- ^ Loh HS, Watters K & Wilson CW (1 November 1973). "The Effects of Aspirin on the Metabolic Availability of Ascorbic Acid in Human Beings". J Clin Pharmacol 13 (11): 480–6. PMID 4490672. http://jcp.sagepub.com/cgi/content/abstract/13/11/480.
- ^ Basu TK (1982). "Vitamin C-aspirin interactions". Int J Vitam Nutr Res Suppl 23: 83–90. PMID 6811490.
- ^ Ioannides C, Stone AN, Breacker PJ & Basu TK (1982). "Impairment of absorption of ascorbic acid following ingestion of aspirin in guinea pigs". Biochem Pharmacol 31 (24): 4035–8. doi:10.1016/0006-2952(82)90652-9. PMID 6818974.
- ^ Reynolds EF (ed) (1982). Aspirin and similar analgesic and anti-inflammatory agents. Martindale, The Extra Pharmacopoeia 28 Ed, 234-82.
- ^ Palleros, Daniel R. (2000). Experimental Organic Chemistry. New York: John Wiley & Sons. p. 494. ISBN 0-471-28250-2.
- ^ Barrans, Richard. "Aspirin Aging". Newton BBS. http://www.newton.dep.anl.gov/askasci/chem00/chem00314.htm. Retrieved 2008-05-08.
- ^ Carstensen, J.T.; F Attarchi and XP Hou (1985). "Decomposition of aspirin in the solid state in the presence of limited amounts of moisture". Journal of Pharmaceutical Sciences 77 (4): 318–21. doi:10.1002/jps.2600770407. PMID 4032246.
- ^ "Acetylsalicylic acid". Jinno Laboratory, School of Materials Science, Toyohashi University of Technology. March 4, 1996. http://chrom.tutms.tut.ac.jp/JINNO/DRUGDATA/07acetylsalicylic_acid.html. Retrieved 2007-09-07.
- ^ Peddy Vishweshwar, Jennifer A. McMahon, Mark Oliveira, Matthew L. Peterson, and Michael J. Zaworotko (2005). "The Predictably Elusive Form II of Aspirin". J. Am. Chem. Soc. 127 (48): 16802–16803. doi:10.1021/ja056455b. PMID 16316223.
- ^ Andrew D. Bond, Roland Boese, Gautam R. Desiraju (2007). "On the Polymorphism of Aspirin: Crystalline Aspirin as Intergrowths of Two "Polymorphic" Domains". Angewandte Chemie International Edition 46 (4): 618–622. doi:10.1002/anie.200603373. PMID 17139692.
- ^ John Robert Vane (1971). "Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs". Nature - New Biology 231 (25): 232–5. PMID 5284360.
- ^ Vane JR, Botting RM (2003). "The mechanism of action of aspirin" (PDF). Thromb Res 110 (5–6): 255–8. doi:10.1016/S0049-3848(03)00379-7. PMID 14592543. http://www.eao.chups.jussieu.fr/polys/certifopt/saule_coxib/theme/1vane2003.pdf.
- ^ "Aspirin in Heart Attack and Stroke Prevention". American Heart Association. http://www.americanheart.org/presenter.jhtml?identifier=4456. Retrieved 2008-05-08.
- ^ Tohgi, H; S Konno, K Tamura, B Kimura and K Kawano (1992). "Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin". Stroke 23 (10): 1400–1403. PMID 1412574.
- ^ Somasundaram, S. et al.; Sigthorsson, G; Simpson, RJ; Watts, J; Jacob, M; Tavares, IA; Rafi, S; Roseth, A et al. (2000). "Uncoupling of intestinal mitochondrial oxidative phosphorylation and inhibition of cyclooxygenase are required for the development of NSAID-enteropathy in the rat". Aliment Pharmacol Ther 14 (5): 639–650. doi:10.1046/j.1365-2036.2000.00723.x. PMID 10792129.
- ^ Paul-Clark, Mark J.; Cao, Thong van; Moradi-Bidhendi, Niloufar; Cooper, Dianne & Gilroy, Derek W. (2004). "15-epi-lipoxin A4–mediated Induction of Nitric Oxide Explains How Aspirin Inhibits Acute Inflammation". J. Exp. Med. 200 (1): 69–78. doi:10.1084/jem.20040566. PMC 2213311. PMID 15238606. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2213311.
- ^ McCarty, M. F.; Block, K. I. (2006). "Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy". Integr Cancer Ther. 5 (3): 252–268. doi:10.1177/1534735406291499. PMID 16880431.
- ^ Caviezel, F; Cattaneo, AG; Tell, A; Corino, T; Mascherpa, M (1983). "The effect of acetylsalicylic acid and diclofenac on stimulated growth hormone and prolactin secretion in humans". International journal of clinical pharmacology, therapy, and toxicology 21 (10): 502–4. PMID 6642786.
- ^ Ramey, JN; Burrow, GN; Spaulding, SW; Donabedian, RK; Speroff, L; Frantz, AG (1976). "The effect of aspirin and indomethacin on the TRH response in man". The Journal of clinical endocrinology and metabolism 43 (1): 107–14. doi:10.1210/jcem-43-1-107. PMID 820703.
- ^ a b Nye EJ, Hockings GI, Grice JE, Torpy DJ, Walters MM, Crosbie GV, Wagenaar M, Cooper M, Jackson RV (1997). "Aspirin inhibits vasopressin-induced hypothalamic-pituitary-adrenal activity in normal humans". J. Clin. Endocrinol. Metab. 82 (3): 812–7. doi:10.1210/jc.82.3.812. PMID 9062488.
- ^ Hockings GI, Grice JE, Crosbie GV, Walters MM, Jackson AJ, Jackson RV (1993). "Aspirin increases the human hypothalamic-pituitary-adrenal axis response to naloxone stimulation". J. Clin. Endocrinol. Metab. 77 (2): 404–8. doi:10.1210/jc.77.2.404. PMID 8393884.
- ^ Ferguson, RK; Boutros, AR (1970-08-17). "Death following self-poisoning with aspirin". Journal of the American Medical Association 213 (7): 1186–8. doi:10.1001/jama.213.7.1186. PMID 5468267.
- ^ Kaufman, FL; Dubansky, AS (1970-04). "Darvon poisoning with delayed salicylism: a case report". Pediatrics 49 (4): 610–1. PMID 5013423.
- ^ a b c Levy, G; Tsuchiya, T (1972-09-31). "Salicylate accumulation kinetics in man". New England Journal of Medicine 287 (9): 430–2. doi:10.1056/NEJM197208312870903. PMID 5044917.
- ^ 2,3-Dihydroxybenzoic acid is a product of human aspirin metabolism. Martin Grootveld and Barry Halliwell, Biochemical Pharmacology, Volume 37, Issue 2, 15 January 1988, pages 271-280, doi:10.1016/0006-2952(88)90729-0
- ^ Hartwig, Otto H (1983-11-14). "Pharmacokinetic considerations of common analgesics and antipyretics". American Journal of Medicine 75 (5A): 30–7. doi:10.1016/0002-9343(83)90230-9. PMID 6606362.
- ^ Done, AK (1960-11). "Salicylate intoxication. Significance of measurements of salicylate in blood in cases of acute ingestion". Pediatrics 26: 800–7. PMID 13723722.
- ^ Chyka PA, Erdman AR, Christianson G, Wax PM, Booze LL, Manoguerra AS, Caravati EM, Nelson LS, Olson KR, Cobaugh DJ, Scharman EJ, Woolf AD, Troutman WG; Americal Association of Poison Control Centers; Healthcare Systems Bureau, Health Resources and Services Administration, Department of Health and Human Services. (2007). "Salicylate poisoning: an evidence-based consensus guideline for out-of-hospital management". Clin Toxicol (Phila) 45 (2): 95–131. doi:10.1080/15563650600907140. PMID 17364628.
- ^ Prescott LF, Balali-Mood M, Critchley JA, Johnstone AF, Proudfoot AT (1982). "Diuresis or urinary alkalinisation for salicylate poisoning?". Br Med J (Clin Res Ed) 285 (6352): 1383–6. doi:10.1136/bmj.285.6352.1383. PMC 1500395. PMID 6291695. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1500395.
- ^ Dargan PI, Wallace CI, Jones AL. (2002). "An evidenced based flowchart to guide the management of acute salicylate (aspirin) overdose". Emerg Med J 19 (3): 206–9. doi:10.1136/emj.19.3.206. PMC 1725844. PMID 11971828. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1725844.
- ^ Mary Bellis (2010-06-16). "History of aspirin". Inventors.about.com. http://inventors.about.com/library/inventors/blaspirin.htm. Retrieved 2011-05-11.
- ^ (German) Gerhardt C (1853). "Untersuchungen über die wasserfreien organischen Säuren". Annalen der Chemie und Pharmacie 87: 149–179. doi:10.1002/jlac.18530870107.
- ^ (German) von Gilm H (1859). "Acetylderivate der Phloretin- und Salicylsäure". Annalen der Chemie und Pharmacie 112 (2): 180–185. doi:10.1002/jlac.18591120207.
- ^ (German)Schröder, Prinzhorn, Kraut K (1869). "Uber Salicylverbindungen". Annalen der Chemie und Pharmacie 150 (1): 1–20. doi:10.1002/jlac.18691500102.
- ^ Mahdi, JG; Mahdi, AJ, Mahdi, AJ, Bowen, ID (2006 Apr). "The historical analysis of aspirin discovery, its relation to the willow tree and antiproliferative and anticancer potential.". Cell proliferation 39 (2): 147–55. doi:10.1111/j.1365-2184.2006.00377.x. PMID 16542349.
- ^ Sneader W (2000 Dec 23-30). "The discovery of aspirin: a reappraisal". BMJ 321 (7276): 1591–4. doi:10.1136/bmj.321.7276.1591. PMC 1119266. PMID 11124191. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1119266.
- ^ Jeffreys, Diarmuid (August 11, 2005). Aspirin: The Remarkable Story of a Wonder Drug. Bloomsbury USA. p. 73. ISBN 1582346003.
- ^ Singer, H. (1901). "Ueber Aspirin". Pflügers Archiv: European Journal of Physiology 84 (11-12): 527–546. doi:10.1007/BF01769129.
- ^ Starko, Karen M. (2009). "Salicylates and Pandemic Influenza Mortality, 1918–1919 Pharmacology, Pathology, and Historic Evidence". Clinical Infectious Diseases 49 (9): 1405. doi:10.1086/606060.
- ^ Jeffreys, Aspirin, pp. 136–142 and 151-152
- ^ "Bayer patents aspirin — History.com This Day in History — 3/6/1899". History.com. http://www.history.com/this-day-in-history.do?action=VideoArticle&id=52415. Retrieved 2011-05-11.
- ^ Jeffreys, Aspirin, pp. 212–217
- ^ Jeffreys, Aspirin, pp. 226–231
- ^ Jeffreys, Aspirin, pp. 267–269
- ^ "Treaty of Versailles, Part X, Section IV, Article 298". 1919-06-28. pp. Annex, Paragraph 5. http://en.wikisource.org/wiki/Treaty_of_Versailles/Part_X#Article_298. Retrieved 2008-10-25.
- ^ Mehta, Aalok (2005). "Aspirin". Chemical & Engineering News 83 (25). http://pubs.acs.org/cen/coverstory/83/8325/8325aspirin.html. Retrieved 2008-10-23.
- ^ "The Centenary of Aspirin". Ul.ie. 1999-03-06. http://www.ul.ie/~childsp/CinA/Issue59/TOC43_Aspirin.htm. Retrieved 2011-05-11.
- ^ CBE Style Manual Committee; Huth, Edward J. (1994). Scientific Style and Format: The CBE Manual for Authors, Editors, and Publishers. Cambridge University Press. p. 164. ISBN 9780521471541. http://books.google.com/books?id=PoFJ-OhE63UC&pg=PA164.
- ^ "Aspirin: the versatile drug". CBC News. 2009-05-28. http://www.cbc.ca/health/story/2009/05/28/f-aspirin-studies.html.
- ^ Cheng, Tsung O. (2007). "The History of Aspirin". Texas Heart Institute Journal 34 (3): 392–393. PMC 1995051. PMID 17948100. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1995051.
- ^ Sigma Aldrich. "Aspirin". http://www.sigmaaldrich.com/catalog/ProductDetail.do?lang=en&N4=A2093. Retrieved 13 July 2009.
- ^ British Pharmacopoeia. "Index BP 2009". http://www.pharmacopoeia.co.uk/pdf/2009_index.pdf. Retrieved 13 July 2009.
- ^ Crosby, Janet Tobiassen (2006). "Veterinary Questions and Answers". About.com. http://vetmedicine.about.com/cs/altvetmedgeneral/a/dogcataspirin.htm. Retrieved 2007-09-05.
- ^ Cambridge H, Lees P, Hooke RE, Russell CS (1991). "Antithrombotic actions of aspirin in the horse". Equine Vet J 23 (2): 123–7. doi:10.1111/j.2042-3306.1991.tb02736.x. PMID 1904347.
- ^ Lappin, [edited by] Michael R. (2001). Feline internal medicine secrets. Philadelphia: Hanley & Belfus. p. 160. ISBN 1-56053-461-3.
External links
- NextBio Aspirin Entry
- Interactive 3D-structure of aspirin with detailed x-ray crystal structure data
- The History of Aspirin
- How Aspirin works
- The science behind aspirin
- Take two: Aspirin, New uses and new dangers are still being discovered as aspirin enters its 2nd century. Shauna Roberts, American Chemical Society
- Ling, Greg (2005). "Aspirin". How Products are Made. 1. Thomson Gale. http://www.madehow.com/Volume-1/Aspirin.html.
- U.S. National Library of Medicine: Drug Information Portal - Aspirin
Anti-inflammatory products (M01A) Pyrazolidine/Butylpyrazolidines Ampyrone • Clofezone • Kebuzone • Metamizole • Mofebutazone • Oxyphenbutazone • Phenazone • Phenylbutazone • Sulfinpyrazone • Feprazone •Acetic acid derivatives
and related substancesAceclofenac • Acemetacin • Alclofenac • Bromfenac • Bumadizone • Bufexamac • Diclofenac • Difenpiramide • Etodolac • Fentiazac • Indometacin • Ketorolac • Lonazolac • Oxametacin • Proglumetacin • Sulindac • Tolmetin • Zomepirac • AmfenacOxicams Propionic acid derivatives Alminoprofen • Benoxaprofen • Dexibuprofen • Dexketoprofen • Fenbufen • Fenoprofen • Flunoxaprofen • Flurbiprofen • Ibuprofen • Ibuproxam • Indoprofen • Ketoprofen • Naproxen • Oxaprozin • Pirprofen • Suprofen • Tiaprofenic acidFenamates Coxibs Other Non-steroidal anti-inflammatory drug (NSAID) products (primarily M01A and M02A, also N02BA) Salicylates Aspirin (acetylsalicylic acid) • Aloxiprin • Benorylate • Diflunisal • Ethenzamide • Magnesium salicylate • Methyl salicylate • Salsalate • Salicin • Salicylamide • Sodium salicylateArylalkanoic acids Diclofenac • Aceclofenac • Acemetacin • Alclofenac • Bromfenac • Etodolac • Indometacin • Indometacin farnesil • Nabumetone • Oxametacin • Proglumetacin • Sulindac • Tolmetin2-Arylpropionic acids
(profens)Ibuprofen • Alminoprofen • Benoxaprofen • Carprofen • Dexibuprofen • Dexketoprofen • Fenbufen • Fenoprofen • Flunoxaprofen • Flurbiprofen • Ibuproxam • Indoprofen† • Ketoprofen • Ketorolac • Loxoprofen • Miroprofen • Naproxen • Oxaprozin • Pirprofen • Suprofen • Tarenflurbil • Tiaprofenic acidN-Arylanthranilic acids
(fenamic acids)Pyrazolidine derivatives Oxicams COX-2 inhibitors Celecoxib • Deracoxib‡ • Etoricoxib • Firocoxib‡ • Lumiracoxib† • Parecoxib • Rofecoxib† • Valdecoxib†Sulfonanilides Topically used products Bendazac • Diclofenac • Etofenamate • Felbinac • Flurbiprofen • Ibuprofen • Indometacin • Ketoprofen • Naproxen • Piroxicam • SuprofenCOX-inhibiting nitric oxide donators Others Items listed in bold indicate initially developed compounds of specific groups. †Withdrawn drugs. ‡Veterinary use medications.Analgesics (N02A, N02B) Opioids
See also: Opioids templateOpium & alkaloids thereofSemi-synthetic opium
derivativesSynthetic opioidsAlphaprodine • Anileridine • Butorphanol • Dextromoramide • Dextropropoxyphene • Dezocine • Fentanyl • Ketobemidone • Levorphanol • Methadone • Meptazinol • Nalbuphine • Pentazocine • Propoxyphene • Propiram • Pethidine • Phenazocine • Piminodine • Piritramide • Tapentadol • Tilidine • Tramadol
Pyrazolones Cannabinoids Anilides Non-steroidal
anti-inflammatories
See also: NSAIDs templatePropionic acid classFenoprofen • Flurbiprofen • Ibuprofen# • Ketoprofen • Naproxen • Oxaprozin
Oxicam classAcetic acid classDiclofenac • Indometacin • Ketorolac • Nabumetone • Sulindac • Tolmetin
Celecoxib • Rofecoxib • Valdecoxib • Parecoxib • Lumiracoxib
Anthranilic acid
(fenamate) classMeclofenamate • Mefenamic acid
SalicylatesAspirin (Acetylsalicylic acid)# • Benorylate • Diflunisal • Ethenzamide • Magnesium salicylate • Salicin • Salicylamide • Salsalate • Trisalate • Wintergreen (Methyl salicylate)
Atypical, adjuvant and potentiators,
Metabolic agents and miscellaneousAmitryptiline • Befiradol • Bicifadine • Carisoprodol • Camphor • Cimetidine • Clonidine • Chlorzoxazone • Cyclobenzaprine • Duloxetine • Esreboxetine • Flupirtine • Gabapentin • Glafenine • Hydroxyzine • Ketamine • Menthol • Mephenoxalone • Methocarbamol • Nefopam • Orphenadrine • Pregabalin • Proglumide • Scopolamine • Tebanicline • Trazodone • Gabapentin enacarbil • ZiconotideAcne-treating agents (D10) Antibacterial Keratolytic Anti-inflammatory Antibiotics Hormonal Retinoids Combinations Adapalene/benzoyl peroxide • Benzoyl peroxide/clindamycin • Clindamycin/tretinoin • Erythromycin/isotretinoin • Sulfacetamide/sulfurSalicylic acid • Aspirin • Aloxiprin • Methyl salicylate • Magnesium salicylate • Ethyl salicylate • Bismuth subsalicylate • Sodium salicylate • Salicylamide • Salicin • Benorilate • Salsalate • Ethenzamide • Diflunisal • Trolamine salicylate • Homosalate • Salicylmethylecgonine • Octyl salicylate • Aluminon • Benzyl salicylate • Copper aspirinate • Potassium salicylateCategories:- Aspirin
- Non-steroidal anti-inflammatory drugs
- Antiplatelet drugs
- Benzoic acids
- Equine medications
- Salicylates
- World Health Organization essential medicines
- Acetate esters
Wikimedia Foundation. 2010.