- Lornoxicam
-
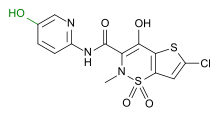
Lornoxicam 
Systematic (IUPAC) name (3E)-6-chloro-3-[hydroxy(pyridin-2-ylamino)methylene]-2-methyl-2,3-dihydro-4H-thieno[2,3-e][1,2]thiazin-4-one 1,1-dioxide Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. Not recommended; contraindicated in months 7–9 Legal status ℞ Prescription only Routes Oral, parenteral Pharmacokinetic data Bioavailability 90–100% Protein binding 99% Metabolism CYP2C9 Half-life 3–4 hours Excretion 2/3 hepatic, 1/3 renal Identifiers ATC code M01AC05 PubChem CID 5282204 DrugBank DB06725 ChemSpider 4445392 
UNII ER09126G7A 
KEGG D01866 
Chemical data Formula C13H10ClN3O4S2 Mol. mass 371.8192 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Lornoxicam (INN, or chlortenoxicam; trade name Xefo, among others) is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic (pain relieving), anti-inflammatory and antipyretic (fever reducing) properties. It is available in oral and parenteral formulations.
Contents
Indications
Lornoxicam is used for the treatment of various types of pain, especially resulting from inflammatory diseases of the joints, osteoarthritis, surgery, sciatica, and other inflammations.[1]
Contraindications
The drug is contraindicated in patients that must not take other NSAIDs, possible reasons including salicylate sensitivity, gastrointestinal bleeding and bleeding disorders, and severe impairment of heart, liver or kidney function. Lornoxicam is not recommended during pregnancy and breastfeeding and is contraindicated during the last third of pregnancy.[1]
Adverse effects
Lornoxicam has side effects similar to other NSAIDs, most commonly mild ones like gastrointestinal disorders (nausea and diarrhea) and headache. Severe but seldom side effects include bleeding, bronchospasms and the extremely rare Stevens–Johnson syndrome.[1]
Pharmacokinetics
Absorption
Lornoxicam is absorbed rapidly and almost completely from the gastro-intestinal tract. Maximum plasma concentrations are achieved after approximately 1 to 2 hours. Food protracts the average time to maximum concentration from 1.5 to about 2.3 hours and can reduce the area under the curve (AUC) by up to 20%.[1]
Distribution
The absolute bioavailability of Lornoxicam is 90–100%. No first-pass effect was observed.[1]
Metabolism
Lornoxicam is found in the plasma in unchanged form and as its hydroxylated metabolite. The hydroxylated metabolite exhibits no pharmacological activity. CYP2C9 has been shown to be the primary enzyme responsible for the biotransformation of the lornoxicam to its major metabolite, 5’-hydroxylornoxicam.[1] Lornoxicam 5’-hydroxylation by the variant CYP2C9*3 and CYP2C9*13 is markedly reduced compared with wild type, both in vitro and in vivo.
Elimination
Approximately 1/2 to 2/3 is eliminated via the liver and 1/3 to 42% (data are inconsistent) via the kidneys as 5’-hydroxylornoxicam.[1]
Mechanism of action
Like other NSAIDs, lornoxicam inhibits prostaglandin biosynthesis by blocking the enzyme cyclooxygenase.
Lornoxicam inhibits both isoforms in the same concentration range, that is, the ratio of COX-1 inhibition to COX-2 inhibition is 1:1. It readily penetrates into the synovial fluid. The AUC ratio of synovial fluid to blood plasma is 0.5 after administration of 4 mg twice daily.
Interactions
Interactions with other drugs are typical of NSAIDs. Combination with vitamin K antagonists like warfarin increases the risk of bleeding. Combination with ciclosporin can lead to reduced kidney function, and to acute renal failure in rare cases. Lornoxicam can also increase the adverse effects of lithium, methotrexate and digoxin and its derivatives. The effect of diuretics, ACE inhibitors and angiotensin II receptor antagonists can be reduced, but this is only relevant in patients with special risks like heart failure. As with piroxicam, cimetidine can increase plasma levels but is unlikely to cause relevant interactions.[2]
References
- ^ a b c d e f g Haberfeld, H, ed (2009) (in German). Austria-Codex (2009/2010 ed.). Vienna: Österreichischer Apothekerverlag. ISBN 3-85200-196-X.
- ^ Klopp, T, ed (2010) (in German). Arzneimittel-Interaktionen (2010/2011 ed.). Arbeitsgemeinschaft für Pharmazeutische Information. ISBN 978-3-85200-207-1.
Anti-inflammatory products (M01A) Pyrazolidine/Butylpyrazolidines Ampyrone • Clofezone • Kebuzone • Metamizole • Mofebutazone • Oxyphenbutazone • Phenazone • Phenylbutazone • Sulfinpyrazone • Feprazone •Acetic acid derivatives
and related substancesAceclofenac • Acemetacin • Alclofenac • Bromfenac • Bumadizone • Bufexamac • Diclofenac • Difenpiramide • Etodolac • Fentiazac • Indometacin • Ketorolac • Lonazolac • Oxametacin • Proglumetacin • Sulindac • Tolmetin • Zomepirac • AmfenacOxicams Propionic acid derivatives Alminoprofen • Benoxaprofen • Dexibuprofen • Dexketoprofen • Fenbufen • Fenoprofen • Flunoxaprofen • Flurbiprofen • Ibuprofen • Ibuproxam • Indoprofen • Ketoprofen • Naproxen • Oxaprozin • Pirprofen • Suprofen • Tiaprofenic acidFenamates Coxibs Other Categories:- Non-steroidal anti-inflammatory drugs
- Thienothiazines
- Pyridines
- Organochlorides
- Amides
Wikimedia Foundation. 2010.