- Ketoprofen
-
Ketoprofen 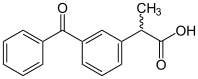
Systematic (IUPAC) name (RS)-2-(3-benzoylphenyl)propanoic acid Clinical data AHFS/Drugs.com monograph MedlinePlus a686014 Pregnancy cat. C (D in 3rd trimester) Legal status Prescription-only medicine Routes Oral, topical, intravenous (veterinary use) Pharmacokinetic data Protein binding 99% Half-life 2-2.5 hours Identifiers CAS number 22071-15-4 
ATC code M01AE03 M01AE17, M02AA10 PubChem CID 3825 DrugBank DB01009 ChemSpider 3693 
UNII 90Y4QC304K 
KEGG D00132 
ChEBI CHEBI:6128 
ChEMBL CHEMBL571 
Chemical data Formula C16H14O3 Mol. mass 254.281 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ketoprofen, (RS)2-(3-benzoylphenyl)-propionic acid (chemical formula C16H14O3) is one of the propionic acid class of non-steroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic effects.[1] It acts by inhibiting the body's production of prostaglandin.
Contents
Available forms
Ketoprofen was available over-the-counter in the United States in the form of 12.5 mg coated tablets (Orudis KT & Actron), but this form has been discontinued. It is available by prescription as 50, 75, 100, 150, and 200 mg capsules.
Ketoprofen is available also as a 2.5% gel for topical application.
Brand names in the US are Orudis and Oruvail. It is available in the UK as Ketoflam and Oruvail, in Finland as Ketorin, Keto, Ketomex, and Orudis'; in France as Profénid, Bi-Profénid and Ketum, in Italy as Ketodol, Fastum Gel, Lasonil, Orudis or Oki, in Poland, Serbia, Slovenia and Croatia as Knavon or Ketonal, in Mexico as Arthril, in Norway as Zon or Orudis, in Russia as ОКИ (OKI) and Ketonal, in Spain as Actron and in Venezuela as Ketoprofeno under an injectable solution of 100 mg and 150 mg capsules.
In Lithuania, ketoprofen is called Ketoprofenum and/or Ketoprofenas. For topical application: the name brands are Fastum with 2.5% (gel) which is over the counter and Ketospray with 10% (liquid spray) which must be prescribed. In Switzerland, an innovative ketoprofen formulation based on Transfersome technology for direct application on the skin above the site to be treated has been approved.
In some countries, the optically pure (S)-enantiomer (dexketoprofen) is available; its trometamol salt is said to be particularly rapidly reabsorbed from the gastrointestinal tract, having a rapid onset of effects.
Indication
Ketoprofen is generally prescribed for arthritis-related inflammatory pains or severe toothaches that result in the inflammation of the gums.
Ketoprofen topical patches are being extensively used for treatment of musculoskeletal pain.[2][3][4] The patches have been shown to provide rapid and sustained delivery to underlying tissues without significantly increasing levels of drug concentration in the blood when compared to the traditional oral administration.[4][5] Ketoprofen undergoes metabolism in the liver via conjugation with glucoronic acid, CYP3A4 and CYP2C9 hydroxylation of the benzoyl ring, and reduction of its keto function.[6][7] Ketoprofen is used for its antipyretic, analgesic, and anti-inflammatory properties by inhibiting cyclooxygenase-1 and -2 (COX-1 and COX-2) enzymes reversibly, which decreases production of pro-inflammatory prostaglandin precursors.[6][8]
Ketoprofen can also be used for treatment of some pain, especially nerve pain like sciatica, post-herpetic neuralgia and referred pain for radiculopathy, in the form of a cream, ointment, liquid, spray, or gel which also contains ketamine and lidocaine, along with other agents which may be useful such as cyclobenzaprine, amitryptiline, acyclovir, gabapentin, orphenadrine and other drugs used as NSAIDs or adjuvant, atypical or potentiators for pain treatment.
Efficacy
The results vary in each case, with varying degrees of the attenuation of the burning, tingling, and/or shooting component of neuralgic pain being quite frequent, along with a modest reduction in overall pain level in some cases. In severe cases and those involving multiple conditions in addition to the cause of the neuralgia, there is no substitute for comprehensive palliative therapy including systemic NSAIDs, opioids, muscle relaxants, intermittent corticosteroids and other agents if needed and adjuncts & potentiators to increase the power of the analgesics in addition to the topical preparation and physical and alternative modalities such as physiotherapy, acupuncture, biofeedback & related modalities, nutritional approaches and many others relevant to each case. Even the addition of the opioid tramadol to topical preparations is not known to be an adequate replacement for systemic opioids in cases where they can be useful and efficacious.
Use in horses and other animals
Ketoprofen is a common NSAID, antipyretic, and analgesic used in horses and other equines.[9] It is also used as a mild painkiller in smaller animals, generally following surgical procedures. It is most commonly used for musculoskeletal pain, joint problems, and soft tissue injury, as well as laminitis. It does not treat the underlying problem, nor does it speed the healing process. It is also used to control fevers and prevent endotoxemia. However, they may mask the symptoms of the underlying problem, and therefore make diagnosis more difficult for a veterinarian.
Uses with other drugs
Ketoprofen should not be used in with other NSAIDs or corticosteroids, as this increases the risk of GI ulceration. It should also be used with caution with other anticoagulants. It is commonly used with omeprazole, sucralfate, and cimetidine to help protect the GI tract.
Administration
Ketoprofen, when administered intravenously, is recommended for a maximum of five days of use. Its analgesic and antipyretic effects begin to occur 1–2 hours following administration. The most common dosage is 1 mg/ lb, once per day, although this dosage may be lowered for ponies, who are most susceptible to NSAID side effects. It is also available as a capsule dosage form and a tablet.
Ecological problems
Recent studies have found that Ketoprofen, like Diclofenac is a veterinary drug causing lethal effects in Red-headed Vultures. Vultures feeding on the carcasses of recently-treated livestock suffer acute kidney failure within days of exposure.[10]
According to research, the vulture population has undergone a sharp decline on the Indian subcontinent, 95% decline in 2004, 99.9% decline as of 2008 due to the use of Diclofenac in animals.[11]
The loss of vultures has had a social impact on the Indian Zoroastrian Parsi community, who traditionally use vultures to dispose of human corpses in a Tower of Silence, but are now compelled to seek alternate methods of disposal.
References
- ^ Kantor, T. G. (1986). "Ketoprofen: a review of its pharmacologic and clinical properties". Pharmacotherapy 6 (3): 93–103. PMID 3526298.
- ^ Mazières, B; Rouanet, S; Guillon, Y; Scarsi, C; Reiner, V (2005). "Topical ketoprofen patch in the treatment of tendinitis: a randomized, double blind, placebo controlled study.". The Journal of rheumatology 32 (8): 1563–70. PMID 16078335.
- ^ Mazières, B (2005). "Topical ketoprofen patch.". Drugs in R&D 6 (6): 337–44. doi:10.2165/00126839-200506060-00003. PMID 16274258.
- ^ a b Sekiya, I; Morito, T; Hara, K; Yamazaki, J; Ju, YJ; Yagishita, K; Mochizuki, T; Tsuji, K et al. (2010). "Ketoprofen Absorption by Muscle and Tendon after Topical or Oral Administration in Patients Undergoing Anterior Cruciate Ligament Reconstruction". AAPS PharmSciTech 11 (1): 154–8. doi:10.1208/s12249-009-9367-2. PMC 2850498. PMID 20087696. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2850498.
- ^ Gayman, MD; Turner, RJ; Cui, M (2008). "Physical Limitations and Depressive Symptoms: Exploring the Nature of the Association". The journals of gerontology. Series B, Psychological sciences and social sciences 63 (4): S219–S228. PMC 2844725. PMID 18689771. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2844725.
- ^ a b Ketoprofen. (n.d.). Millennium Web Catalog. Retrieved February 1, 2010, from http://0-online.lexi.com.library.touro.edu
- ^ Lemke TL, Williams DA, Roche VF, Zito SW. Foyes Principles of Medical Chemistry. 6th ed. Philadelphia: Lippincott Williams and Wilkins; 2008.
- ^ Ketoprofen. (n.d.). Micromedex. Retrieved February 1, 2010, from https://bb-tuc.touro.edu/webapps/portal/frameset.jsp?tab_id=_102_1
- ^ Forney, Barbara C, MS, VMD. Equine Medications, Revised Edition. Blood Horse Publications. Lexington, KY. Copyright 2007.
- ^ http://www.birdlife.org/news/news/2009/12/vultures.html
- ^ http://www.nature.com/nature/journal/v427/n6975/full/nature02317.html
Anti-inflammatory products (M01A) Pyrazolidine/Butylpyrazolidines Ampyrone • Clofezone • Kebuzone • Metamizole • Mofebutazone • Oxyphenbutazone • Phenazone • Phenylbutazone • Sulfinpyrazone • Feprazone •Acetic acid derivatives
and related substancesAceclofenac • Acemetacin • Alclofenac • Bromfenac • Bumadizone • Bufexamac • Diclofenac • Difenpiramide • Etodolac • Fentiazac • Indometacin • Ketorolac • Lonazolac • Oxametacin • Proglumetacin • Sulindac • Tolmetin • Zomepirac • AmfenacOxicams Propionic acid derivatives Alminoprofen • Benoxaprofen • Dexibuprofen • Dexketoprofen • Fenbufen • Fenoprofen • Flunoxaprofen • Flurbiprofen • Ibuprofen • Ibuproxam • Indoprofen • Ketoprofen • Naproxen • Oxaprozin • Pirprofen • Suprofen • Tiaprofenic acidFenamates Coxibs Other Topical products for joint and muscular pain (M02) Anti-inflammatory preparations,
non-steroidsAcetic acid derivativesOtherBenzydamine • Etofenamate • Piroxicam • Felbinac • Bufexamac • Ketoprofen • Bendazac • Naproxen • Ibuprofen • Feprazone • Niflumic acid • Meclofenamic acid • Flurbiprofen • Suxibuzone • Indometacin • NifenazoneCapsaicin derivatives Other Analgesics (N02A, N02B) Opioids
See also: Opioids templateOpium & alkaloids thereofSemi-synthetic opium
derivativesSynthetic opioidsAlphaprodine • Anileridine • Butorphanol • Dextromoramide • Dextropropoxyphene • Dezocine • Fentanyl • Ketobemidone • Levorphanol • Methadone • Meptazinol • Nalbuphine • Pentazocine • Propoxyphene • Propiram • Pethidine • Phenazocine • Piminodine • Piritramide • Tapentadol • Tilidine • Tramadol
Pyrazolones Cannabinoids Anilides Non-steroidal
anti-inflammatories
See also: NSAIDs templatePropionic acid classFenoprofen • Flurbiprofen • Ibuprofen# • Ketoprofen • Naproxen • Oxaprozin
Oxicam classAcetic acid classDiclofenac • Indometacin • Ketorolac • Nabumetone • Sulindac • Tolmetin
Celecoxib • Rofecoxib • Valdecoxib • Parecoxib • Lumiracoxib
Anthranilic acid
(fenamate) classMeclofenamate • Mefenamic acid
SalicylatesAspirin (Acetylsalicylic acid)# • Benorylate • Diflunisal • Ethenzamide • Magnesium salicylate • Salicin • Salicylamide • Salsalate • Trisalate • Wintergreen (Methyl salicylate)
Atypical, adjuvant and potentiators,
Metabolic agents and miscellaneousAmitryptiline • Befiradol • Bicifadine • Carisoprodol • Camphor • Cimetidine • Clonidine • Chlorzoxazone • Cyclobenzaprine • Duloxetine • Esreboxetine • Flupirtine • Gabapentin • Glafenine • Hydroxyzine • Ketamine • Menthol • Mephenoxalone • Methocarbamol • Nefopam • Orphenadrine • Pregabalin • Proglumide • Scopolamine • Tebanicline • Trazodone • Gabapentin enacarbil • ZiconotideCategories:- Non-steroidal anti-inflammatory drugs
- Equine medications
- Aromatic ketones
Wikimedia Foundation. 2010.
