- Oxaprozin
-
Oxaprozin 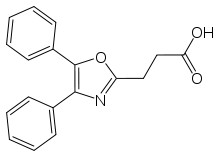
Systematic (IUPAC) name 3-(4,5-diphenyl-1,3-oxazol-2-yl)propanoic acid Clinical data Trade names Daypro AHFS/Drugs.com monograph MedlinePlus a693002 Pregnancy cat. C Legal status ? Routes Oral Pharmacokinetic data Bioavailability 95% Protein binding 99% Metabolism Liver—65% oxidation and 35% glucuronic acid conjugation. 5% are active phenolic metabolites. Half-life 54.9 hours Identifiers CAS number 21256-18-8 ATC code M01AE12 PubChem CID 4614 DrugBank APRD00030 ChemSpider 4453 
UNII MHJ80W9LRB 
KEGG D00463 
ChEMBL CHEMBL1071 
Chemical data Formula C18H15NO3 Mol. mass 293.317 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Oxaprozin, also known as Oxaprozinum, (sold under the names: Daypro, Dayrun, Duraprox) is a non-steroidal anti-inflammatory drug (NSAID),[1] used to relieve the inflammation, swelling, stiffness, and joint pain associated with osteoarthritis and rheumatoid arthritis. Chemically, it is a propionic acid derivative. It is available in 600 mg tablets. Normal adult dosage is 1200 mg daily, not to exceed 1800 mg per day. Safety and efficacy has been established in children over 6 years with juvenile rheumatoid arthritis only, and there is an increased risk of adverse reactions in the elderly population.
References
- ^ Greenblatt DJ, Matlis R, Scavone JM, Blyden GT, Harmatz JS, Shader RI (March 1985). "Oxaprozin pharmacokinetics in the elderly". British journal of clinical pharmacology 19 (3): 373–8. PMC 1463728. PMID 3986088. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1463728.
Anti-inflammatory products (M01A) Pyrazolidine/Butylpyrazolidines Ampyrone • Clofezone • Kebuzone • Metamizole • Mofebutazone • Oxyphenbutazone • Phenazone • Phenylbutazone • Sulfinpyrazone • Feprazone •Acetic acid derivatives
and related substancesAceclofenac • Acemetacin • Alclofenac • Bromfenac • Bumadizone • Bufexamac • Diclofenac • Difenpiramide • Etodolac • Fentiazac • Indometacin • Ketorolac • Lonazolac • Oxametacin • Proglumetacin • Sulindac • Tolmetin • Zomepirac • AmfenacOxicams Propionic acid derivatives Alminoprofen • Benoxaprofen • Dexibuprofen • Dexketoprofen • Fenbufen • Fenoprofen • Flunoxaprofen • Flurbiprofen • Ibuprofen • Ibuproxam • Indoprofen • Ketoprofen • Naproxen • Oxaprozin • Pirprofen • Suprofen • Tiaprofenic acidFenamates Coxibs Other 
This drug article relating to the musculoskeletal system is a stub. You can help Wikipedia by expanding it.
