- Nimesulide
-
Nimesulide 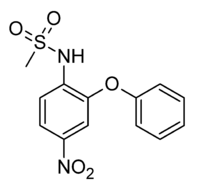
Systematic (IUPAC) name N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide Clinical data AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status Usually prescription only Routes Oral, rectal, topical Pharmacokinetic data Protein binding >97.5% Metabolism Hepatic Half-life 1.8–4.7h Excretion Renal (50%), fecal (29%) Identifiers CAS number 51803-78-2 
ATC code M01AX17 M02AA26 PubChem CID 4495 DrugBank DB04743 ChemSpider 4339 
UNII V4TKW1454M 
KEGG D01049 
ChEBI CHEBI:44445 
ChEMBL CHEMBL56367 
Chemical data Formula C13H12N2O5S Mol. mass 308.311 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Nimesulide is a relatively COX-2 selective, non-steroidal anti-inflammatory drug (NSAID) with analgesic and antipyretic properties. Its approved indications are the treatment of acute pain, the symptomatic treatment of osteoarthritis and primary dysmenorrhoea in adolescents and adults above 12 years old. Due to concerns about the risk of hepatotoxicity, nimesulide has been withdrawn from market in many countries.[1]
History
It was launched in Italy for the first time as Aulin and Mesulid in 1985 and is presently available in more than 50 countries worldwide, among others France, Portugal, Greece, Switzerland, Belgium, Mexico and Brazil. Nimesulide has never been filed for Food and Drug Administration (FDA) evaluation in the United States, where it is not marketed.[2]
European Medicines Agency reports favourable benefit/risk ratio
On August 1, 2003 the Committee for Proprietary Medicinal Products (CPMP) of the European Medicines Agency (EMA) reported that the benefit/risk profile of nimesulide containing medicinal products (Aulin, Mesulide, Nimed and associated product names) for systemic and topical use is favourable and that Marketing Authorisations should be maintained/granted. The CPMP recommended to restrict the use of nimesulide to the indications of treatment of acute pain, symptomatic treatment of painful osteoarthritis and primary dysmenorrhoea for the systemic formulations and symptomatic relief of pain associated with sprains and acute tendinitis for the topical formulation.[3]
Alembic Ltd. issued a circular asking wholesalers and retailers to withdraw all stocks of Nimegesic Drops (a pediatric dosage form of nimesulide) in 2003, consistent with the fact that nimesulide is, like most NSAIDs, not indicated in children.[4]
Central Drugs Standard Control Organization of India bans Nimesulide
Several reports have been made of adverse drug reactions in India.[5][6][7][8] On Feb 12, 2011, Express India reported that the Union Ministry of Health and Family Welfare had finally decided to ban the pediatric use of the analgesic, Nimesulide suspension. From 2011 onwards, it has been totally banned in India.
Irish Medicines Board (IMB) suspends Nimesulide containing drugs (15 May 2007)
The Irish Medicines Board (IMB) has decided to suspend Nimesulide from the Irish market and refer it to the EU Committee for Human Medicinal Products (CHMP) for a review of its benefit/risk profile. The decision is due to the reporting of six (6) cases of potentially related liver failures to the IMB by the National Liver Transplant Unit, St Vincent Hospital. These cases occurred in the period from 1999 to 2006.[9]
Singapore Health Science Authority suspends Nimesulide containing drugs
Pending review of the drug's safety by the EMA, nimesulide has been suspended with immediate effect (June 15, 2007)[10][11]
EMA confirms the positive benefit/risk ratio
On September 21, 2007 the EMA released a press release on their review on the liver-related safety of nimesulide. The EMA has concluded that the benefits of these medicines outweigh their risks, but that there is a need to limit the duration of use to ensure that the risk of patients developing liver problems is kept to a minimum. Therefore the EMA has limited the use of systemic formulations (tablets, solutions, suppositories) of nimesulide to 15 days.[12]
RTÉ's Prime Time Investigates
On December 3, 2007 Ireland's RTÉ aired an investigative programme highlighting the deadly side effects of Nimesulide and how it has been linked to over 300 cases of liver disease throughout Europe.
Bribes allegedly paid in Italy to spare nimesulide from official scrutiny
In May 2008, Italy's leading daily paper Corriere della Sera and other media outlets reported that a top-ranking official at Italy's medicines agency AIFA had been filmed by police while accepting bribes from employees of pharmaceutical companies.[13][14] The money was allegedly being paid to ensure that certain drugs (nimesulide-containing Aulin being the most prominent) would be spared scrutiny from the drugs watchdog. The investigation had started in 2005 following suspicions that some AIFA drug tests had been faked. Eight arrests were made. Following this, concerns about nimesulide hepatotoxicity became more widely reported by the Italian media. A government minister ordered an inquiry. Presently[when?], nimesulide can be bought carrying a prescription from a physician, that is kept as a receipt at the chemist shop, nominally allowing strong control over selling.
The original manufacturer of Nimesulide is Helsinn Healthcare SA, Switzerland, which acquired the rights for the drug in 1976. After the patent protection had terminated, a number of other companies have started production and marketing of Nimesulide.
Prohibition in several European countries
Nimesulide has been prohibited in several other European countries based on the serious hepatotoxicity that has been suspected of causing several hundred deaths. In 2002, Finland and Spain withdrew nimesulide from the market following reports of serious liver damage. Cases including 2 deaths had also been reported in France at the time. Ireland decided to withdraw nimesulide from the market in 2007. [15]
After serious medical problems including deaths from the food supplement "Fortodol", analysis demonstrated that in several instances this "food supplement from natural products", declared to contain turmeric and phenyl alanine, contained therapeutic doses of Nimesulide. This was thus probably the reason for the serious side effects of this preparation.[16] Identical or similar preparations containing turmeric tainted with nimesulide have been marketed in Denmark, Finland and in the UK under the brand name "Miradin".[17]
In 2010 Kurt Donsbach, the inventor and manufacturer of Fortodols content, pleaded guilty to selling misbranded drugs. Providing supplements with Nimesulide was one of the charges he admitted to.[18]
Risk of serious liver damage
The International Society of Drug Bulletins (ISDB) deems it unacceptable that nimesulide has been allowed to remain on European and some other markets in the world. This non-steroidal anti-inflammatory drug (NSAID) offers no therapeutic advantage or better gastrointestinal safety compared with other NSAIDs, whereas it exposes patients to a higher risk of fatal hepatic disorders.
Nimesulide has never been approved for use in countries like USA, UK, Canada, Australia New Zealand, Japan and other countries in view of concerns over its safety profile. Ireland and Singapore decided to withdraw nimesulide from the market in 2007.[15]
The Therapeutics Initiative website mentions: "The European Medicines Agency has confirmed the hepatic risks associated with nimesulide in 2007, but merely limited the duration of treatment, leaving patients exposed to an unjustifiable fatal risk. These half-hearted measures are all the more unacceptable since numerous other available NSAIDs are just as effective and less dangerous."[15]
Availability
It is available in a variety of forms: tablets, powder for dissolution in water, suppositories and topical gel (Sulidin® Gel).
A recent evaluation from EMEA (the European Medicines Agency) concluded that the overall benefit/risk profile of nimesulide is favourable and in line with that of the other NSAIDs (such as for example, diclofenac, ibuprofen, naproxen).
Trade names
Nimesulide is available through the world as original product with the following trademarks: Sulide, Nimalox, Mesulid, Nilsid (Egypt); Aulin, Ainex, Drexel, Donulide, Edrigyl, Enetra, Eskaflam, Heugan, Mesulid, Minapon, NeRelid, Nexen, Nidolon, Nilden (Mexico); Nimed, Nimedex, Nimesil, Nimulid, Nimutab, Nimdase, Nise (Russia), Nisulid, Nodard Plus, Nicip, Nimcap (India); Novolid, Pain Lock (VIP Pharma India); Relmex (Ecuador); Remisid (Ukraine); Scaflam, Scaflan, Sulidin® (Turkey); Modact-IR (Pakistan);[19] Sulidene and Zolan for veterinary use. Many generic and copy-products also exist (Coxtral, Lusemin, Medicox, Nidol, Nimalox, Nimesil, Nimotas, Nimulid, Nizer, Sorini, Ventor, Vionim, Neolide, Willgo among others).
Pharmacokinetics
Nimesulide is rapidly absorbed following oral administration.[20]
Nimesulide undergoes extensive biotransformation, mainly to 4-hydroxynimesulide (which also appears to be biologically active).[20]
Food, gender and advanced age have negligible effects on nimesulide pharmacokinetics.[20]
Moderate renal impairment does not necessitate dosage adjustment while patients with severe renal impairment or hepatic impairment are contraindicated.[21]
Nimesulide has a relatively rapid onset of action, with meaningful reductions in pain and inflammation observed within 15 minutes from drug intake.[22][23] As many as almost 498 million patients have been treated with nimesulide from its launch until today.[citation needed]
The therapeutic effects of Nimesulide are the result of its complete mode of action which targets a number of key mediators of the inflammatory process such as: COX-2 mediated prostaglandins, free radicals, proteolytic enzymes and histamine.[22] Clinical evidence is available to support a particularly good profile in terms of gastrointestinal tolerability.[24]
As all anti-inflammatory drugs, it should be taken in compliance with the recommendations included in the patient leaflet.
Side effects
Like most drugs in NSAID category, nimesulide is known to be hepatotoxic (damaging to the liver) in rare but unpredictable cases and should be taken with care. The patient information leaflet informs that the use of nimesulide in children under the age of 12 is contraindicated.
The drug has certain side effects, that can affect individuals in different ways. The following are some of the side effects, that are often associated with the drug:
- Diarrhea
- Vomiting
- Skin rash
- Pruritis
- Dizziness
- Headache
- Bitterness in mouth
Women should use the drug with caution during lactation and it is contraindicated during pregnancy.[25]
References
- ^ http://www.medindia.net/news/Unsafe-Drugs-Banned-79989-1.htm
- ^ Traversa G, Bianchi C, Da Cas R, Abraha I, Menniti-Ippolito F, Venegoni M (July 2003). "Cohort study of hepatotoxicity associated with nimesulide and other non-steroidal anti-inflammatory drugs". BMJ 327 (7405): 18–22. doi:10.1136/bmj.327.7405.18. PMC 164233. PMID 12842950. http://www.bmj.com/content/327/7405/18.full.
- ^ European Commission CPMP favourable opinion on nimesulide
- ^ The end begins
- ^ Safety of nimesulide. CD ROM, Appropriate Use of Antipyretics / Analgesics in Children, Health Informatics, New Delhi, 2004.
- ^ Rahman SZ, Khan RA (2004). "Is nimesulide safe in a cardiovascular-Compromised patient?". Indian J Pharmacol 36: 252–3.
- ^ Khan RA, Rahman SZ (2004). "A Case Report on Nimesulide and its Relation with Angina". J Pharmacovigilance Drug Safety 1: 19–21.
- ^ Khan RA, Rahman SZ (2004). "Nimesulide Induced Coronary Artery Insufficiency – A Case Report". J Pharmacovigilance Drug Safety 1: 11–3.
- ^ IMB Announces Immediate Suspension of the Marketing of Medicines Containing Nimesulide
- ^ Channelnewsasia.com
- ^ http://www.hsa.gov.sg/docs/HSAPressRelease_HSASuspendsSalesOfProductsContainingNimesulide_15Jun07.pdf
- ^ EMA press release on nimesulide September 2007
- ^ «Mazzette per evitare i controlli sull'Aulin». Mario Pappagallo, Corriere della Sera, 23 May 2008
- ^ Italian medicines agency officials arrested in corruption probe. Manufacturing Chemist
- ^ a b c http://www.ti.ubc.ca/nimesulide-must-be-withdrawn-worldwide-due-serious-liver-damage
- ^ http://www.lakemedelsverket.se/Tpl/NewsPage____8325.aspx (Swedish)
- ^ http://www.food.gov.uk/enforcement/alerts/2009/mar/miradinfortodol
- ^ Bonita Man Pleads Guilty To Posing As Doctor. Kurt Donsbach, 75, Faces Up To Year In Jail, 13 Dec. 2010, San Diego 10 News.com
- ^ [1]
- ^ a b c Bernareggi A (October 1998). "Clinical pharmacokinetics of nimesulide". Clin Pharmacokinet 35 (4): 247–74. PMID 9812177. http://content.wkhealth.com/linkback/openurl?issn=0312-5963&volume=35&issue=4&spage=247.
- ^ Microsoft Word - opnh.P.Nimesulide .EMEA-CPMP-3086-03-en-Final.doc
- ^ a b Rainsford KD (June 2006). "Nimesulide – a multifactorial approach to inflammation and pain: scientific and clinical consensus". Curr Med Res Opin 22 (6): 1161–70. doi:10.1185/030079906X104849. PMID 16846549. http://informahealthcare.com/doi/abs/10.1185/030079906X104849.
- ^ Bianchi M, Broggini M (2003). "A randomised, double-blind, clinical trial comparing the efficacy of nimesulide, celecoxib and rofecoxib in osteoarthritis of the knee". Drugs 63 (Suppl 1): 37–46. PMID 14506910. http://content.wkhealth.com/linkback/openurl?issn=0012-6667&volume=63&issue=&spage=37.
- ^ Laporte JR, Ibáñez L, Vidal X, Vendrell L, Leone R (2004). "Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents". Drug Saf 27 (6): 411–20. PMID 15144234. http://content.wkhealth.com/linkback/openurl?issn=0114-5916&volume=27&issue=6&spage=411.
- ^ http://www.pharmaceutical-drug-manufacturers.com/pharmaceutical-drugs/nimesulide.html
External links
- nimesulide.net, by pharmaceutical company Helsinn Healthcare
Anti-inflammatory products (M01A) Pyrazolidine/Butylpyrazolidines Ampyrone • Clofezone • Kebuzone • Metamizole • Mofebutazone • Oxyphenbutazone • Phenazone • Phenylbutazone • Sulfinpyrazone • Feprazone •Acetic acid derivatives
and related substancesAceclofenac • Acemetacin • Alclofenac • Bromfenac • Bumadizone • Bufexamac • Diclofenac • Difenpiramide • Etodolac • Fentiazac • Indometacin • Ketorolac • Lonazolac • Oxametacin • Proglumetacin • Sulindac • Tolmetin • Zomepirac • AmfenacOxicams Propionic acid derivatives Alminoprofen • Benoxaprofen • Dexibuprofen • Dexketoprofen • Fenbufen • Fenoprofen • Flunoxaprofen • Flurbiprofen • Ibuprofen • Ibuproxam • Indoprofen • Ketoprofen • Naproxen • Oxaprozin • Pirprofen • Suprofen • Tiaprofenic acidFenamates Coxibs Other Nabumetone • Glucosamine • Benzydamine • Glycosaminoglycan • Magnesium salicylate • Proquazone • Superoxide dismutase/Orgotein • Nimesulide • Diacerein • Tenidap • Oxaceprol • Chondroitin sulfateCategories:- Non-steroidal anti-inflammatory drugs
- Antipyretics
- Analgesics
- Phenol ethers
- Nitrobenzenes
Wikimedia Foundation. 2010.

