- Omeprazole
-
Omeprazole 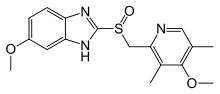
Systematic (IUPAC) name (RS)-6-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl) methylsulfinyl)-1H-benzo[d]imidazole Clinical data Licence data US FDA:link Pregnancy cat. B3(AU) C(US) Legal status Prescription Only (S4) (AU) POM (UK) OTC (US) Routes Oral, IV Pharmacokinetic data Bioavailability 35–76%[1][2] Protein binding 95% Metabolism Hepatic (CYP2C19, CYP3A4) Half-life 1 – 1.2 hours Excretion 80% Renal
20% FaecalIdentifiers CAS number 73590-58-6 
ATC code A02BC01 PubChem CID 4594 DrugBank APRD00446 ChemSpider 4433 
UNII KG60484QX9 
KEGG D00455 
ChEBI CHEBI:7772 
ChEMBL CHEMBL1503 
Chemical data Formula C17H19N3O3S Mol. mass 345.4 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Omeprazole (INN) (
 /oʊˈmɛprəzoʊl/) is a proton pump inhibitor used in the treatment of dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD), laryngopharyngeal reflux (LPR) and Zollinger–Ellison syndrome. Omeprazole is one of the most widely prescribed drugs internationally and is available over the counter in some countries.
/oʊˈmɛprəzoʊl/) is a proton pump inhibitor used in the treatment of dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD), laryngopharyngeal reflux (LPR) and Zollinger–Ellison syndrome. Omeprazole is one of the most widely prescribed drugs internationally and is available over the counter in some countries.Contents
Medical uses
See also: Proton pump inhibitorUse in Helicobacter pylori eradication
Omeprazole is combined with the antibiotics clarithromycin and amoxicillin (or metronidazole in penicillin-hypersensitive patients) in the 7–14 day eradication triple therapy for Helicobacter pylori. Infection by H. pylori is the causative factor in the majority of peptic and duodenal ulcers.[3]
Adverse effects
Some of the most frequent side effects of omeprazole (experienced by over 1% of those taking the drug) are headache, diarrhea, abdominal pain, nausea, dizziness, trouble awakening and sleep deprivation, although in clinical trials the incidence of these effects with omeprazole was mostly comparable to that found with placebo.[4] Other side effects may include iron and vitamin B12 deficiency, although there is very little evidence to support this.[5]
Proton pump inhibitors may be associated with a greater risk of fractures[6][7] and Clostridium difficile-associated diarrhea.[5][8] Patients are frequently administered the drugs in intensive care as a protective measure against ulcers, but this use is also associated with a 30% increase in occurrence of pneumonia.[5][9] The risk of community-acquired pneumonia may also be higher in people taking PPIs.[5]
Since their introduction, proton pump inhibitors (especially omeprazole) have been associated with several cases of acute tubulointerstitial nephritis, an inflammation of the kidneys that often occurs as an adverse drug reaction.[5][10][11]
PPI use has also been associated with fundic gland polyposis.[12]
Interactions
Omeprazole is a competitive inhibitor of the enzymes CYP2C19 and CYP2C9, and may therefore interact with drugs that depend on them for metabolism, such as diazepam, escitalopram, and warfarin; the concentrations of these drugs may increase if they are used concomitantly with omeprazole.[13] Clopidogrel (Plavix) is an inactive prodrug that partially depends on CYP2C19 for conversion to its active form; inhibition of CYP2C19 blocks the activation of clopidogrel, thus reducing its effects and potentially increasing the risk of stroke or heart attack in people taking clopidogrel to prevent these events.[14][15] Omeprazole is also a competitive inhibitor of p-glycoprotein, as are other PPIs.[16]
Drugs that depend on stomach pH for absorption may interact with omeprazole; drugs that depend on an acidic environment (such as ketoconazole or atazanavir) will be poorly absorbed, whereas acid-labile antibiotics (such as erythromycin) will be absorbed to a greater extent than normal due to the more alkaline environment of the stomach.[13]
St. John's wort (Hypericum perforatum) and Gingko biloba significantly reduce plasma concentrations of omeprazole through induction of CYP3A4 and CYP2C19.[17]
Pharmacokinetics
The absorption of omeprazole takes place in the small intestine and is usually completed within 3–6 hours. The systemic bioavailability of omeprazole after repeated dose is about 60%.
Omeprazole bioavailability is significantly impaired by the presence of food and, therefore, patients should be advised to take omeprazole with a glass of water on an empty stomach (i.e., fast for at least 60 minutes before taking omeprazole). Additionally, most sources recommend that after taking omeprazole at least 30 minutes should be allowed to elapse before eating[18][19] (at least 60 minutes for immediate-release omeprazole plus sodium bicarbonate products, such as Zegerid[20]), though some sources say that with delayed-release forms of omeprazole it is not necessary to wait before eating after taking the medication.[21] Plasma protein binding is about 95%.
Omeprazole is completely metabolized by the cytochrome P450 system, mainly in the liver. Identified metabolites are the sulfone, the sulfide and hydroxy-omeprazole, which exert no significant effect on acid secretion. About 80% of an orally given dose is excreted as metabolites in the urine and the remainder is found in the feces, primarily originating from bile secretion.
Measurement in body fluids
Omeprazole may be quantitated in plasma or serum to monitor therapy or to confirm a diagnosis of poisoning in hospitalized patients. Plasma omeprazole concentrations are usually in a range of 0.2–1.2 mg/L in persons receiving the drug therapeutically via the oral route and 1–6 mg/L in victims of acute overdosage. Enantiomeric chromatographic methods are available to distinguish esomeprazole from racemic omeprazole.[22][23]
Chemistry
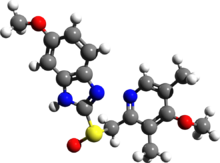
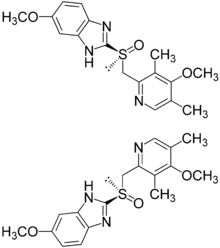 (S)- and (R)-enantiomers of omeprazole
(S)- and (R)-enantiomers of omeprazole
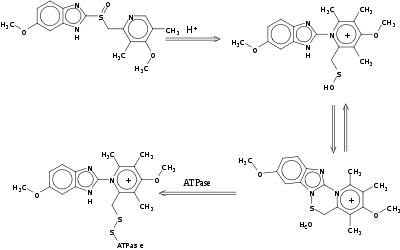
Omeprazole is a racemate. It contains a tricoordinated sulfur in a pyramidal structure and therefore can exist in equal amounts of both the (S)- and (R)-enantiomers. In the acidic conditions of the stomach, both are converted to achiral products,(a sulphenic acid and a sulphenamide configurations) which reacts with a cysteine group in H+/K+ ATPase, thereby inhibiting the ability of the parietal cells to produce gastric acid.
Facing the loss of patent protection and competition from generic drug manufacturers, AstraZeneca developed and heavily marketed esomeprazole (Nexium) as a replacement in 2001.[when?] Esomeprazole is the eutomer [(S)-enantiomer] in the pure form, not a racemate as omeprazole.
Omeprazole undergoes a chiral shift in vivo which converts the inactive (R)-enantiomer to the active (S)-enantiomer doubling the concentration of the active form.[24] This chiral shift is accomplished by the CYP2C19 isozyme of cytochrome P450, which is not found equally in all human populations. Those who do not metabolize the drug effectively are called "poor metabolizers." The proportion of the poor metabolizer phenotype varies widely between populations, from 2–2.5% in African-Americans and white Americans to >20% in Asians; several pharmacogenomics studies have suggested that PPI treatment should be tailored according to CYP2C19 metabolism status.[25]
History
Omeprazole was first marketed in the U.S. in 1989 by AstraZeneca under the brand names Losec and Prilosec. An over the counter brand, Prilosec OTC, is available without prescription in the US for treatment of heartburn. It is now also available from generic manufacturers under various brand names.
AstraZeneca markets omeprazole as Losec, Antra, Gastroloc, Mopral, Omepral, and Prilosec. Omeprazole is marketed as Zegerid by Santarus, Prilosec OTC by Procter & Gamble and Zegerid OTC by Schering-Plough and as Segazole by Star Laboratories in Pakistan.[26][27] In India it is available as OMEZ (Farhaad). In Bangladesh, it is made and marketed by Beacon Pharmaceuticals Ltd. under the brand name Xelopes. Also Healthcare Pharmaceuticals Ltd. marketed omeprazole under the brand name Opal. In Bangladesh Apex Pharma also markets omeprazole under the brand name Aspra. In Argentina it is made by Bago Laboratories S.A. and available there and in Ecuador as Ulcozol. In Indonesia Darya-Varia Laboratories marketed omeprazole as Ozid. In Brazil, omeprazole is produced by Multilab under the name Lozeprel. In Spain it is produced by Cantabria Pharma S.L. under the name emeprotón. In Bangladesh, Eskayef Bangladesh Limited also marketed omeprazole under the brand name Losectil.
In 1990, at the request of the U.S. Food and Drug Administration (FDA), the brand name Losec was changed to Prilosec to avoid confusion with the diuretic Lasix (furosemide).[28] Unfortunately, the new name has led to confusion between omeprazole (Prilosec) and fluoxetine (Prozac), an antidepressant.[28]
Dosage forms
 Package of Losec (Omeprazole) 20 mg, purchased in Hong Kong
Package of Losec (Omeprazole) 20 mg, purchased in Hong Kong
Omeprazole is available as tablets and capsules (containing omeprazole or omeprazole magnesium) in strengths of 10 mg, 20 mg, 40 mg, and in some markets 80 mg; and as a powder (omeprazole sodium) for intravenous injection. Most oral omeprazole preparations are enteric-coated, due to the rapid degradation of the drug in the acidic conditions of the stomach. This is most commonly achieved by formulating enteric-coated granules within capsules, enteric-coated tablets, and the multiple-unit pellet system (MUPS).
It is also available for use in injectable form (I.V.) in Europe, but not in the U.S. The injection pack is a combination pack consisting of a vial and a separate ampule of reconstituting solution. Each 10 ml clear glass vial contains a white to off-white lyophilised powder consisting of omeprazole sodium 42.6 mg equivalent to 40 mg of omeprazole.
Multiple unit pellet system
Omeprazole tablets manufactured by AstraZeneca (notably Losec/Prilosec) are formulated as a "multiple unit pellet system" (MUPS). Essentially, the tablet consists of extremely small enteric-coated granules (pellets) of the omeprazole formulation inside an outer shell. When the tablet is immersed in an aqueous solution, as happens when the tablet reaches the stomach, water enters the tablet by osmosis. The contents swell from water absorption causing the shell to burst, releasing the enteric-coated granules. For most patients, the multiple-unit pellet system is of no advantage over conventional enteric-coated preparations. Patients for which the formulation is of benefit include those requiring nasogastric tube feeding and those with difficulty swallowing (dysphagia) because the tablets can be mixed with water ahead of time, releasing the granules into a slurry form, which is easier to pass down the feeding tube or to swallow than the pill.[citation needed]
The granules are manufactured in a fluid air bed system. Sugar spheres in suspension are sequentially sprayed with aqueous suspensions of omeprazole, a protective layer, an enteric coating and an outer layer to reduce granule aggregation. The granules are mixed with other excipients and compressed into tablets. Finally, the tablets are film-coated to improve the stability and appearance of the preparation.
Immediate release formulation
In June 2004 the FDA approved an immediate release preparation of omeprazole and sodium bicarbonate that does not require an enteric coating. This preparation employs sodium bicarbonate as a buffer to protect omeprazole from gastric acid degradation. This allows for the production of chewable tablets. This combination preparation is marketed in the United States by Santarus under the brand name Zegerid. Zegerid is marketed as capsules, chewable tablets, and powder for oral suspension. Zegerid is most useful for those patients who suffer from nocturnal acid breakthrough (NAB) or those patients who desire immediate relief. In India it is marketed by Dr. Reddy's Laboratories as powder formulation with the brand name OMEZ-INSTA. It is reported to have additional benefits with patients suffering from alcoholic gastritis and life-style associated gastritis.
See also
References
- ^ Prilosec Prescribing Information. AstraZeneca Pharmaceuticals.
- ^ Vaz-Da-Silva, M; Loureiro, AI; Nunes, T; Maia, J; Tavares, S; Falcão, A; Silveira, P; Almeida, L et al. (2005). "Bioavailability and bioequivalence of two enteric-coated formulations of omeprazole in fasting and fed conditions". Clin Drug Investig 25 (6): 391–9. doi:10.2165/00044011-200525060-00004. PMID 17532679. http://www.medscape.com/viewarticle/508018.
- ^ "What Causes Ulcers?". MedicineNet. 9 October 2009.
- ^ "Prilosec Side Effects & Drug Interactions". RxList.com. 2007. http://www.rxlist.com/cgi/generic/omepra_ad.htm. Retrieved 2007-06-16.
- ^ a b c d e Madanick RD (Jan 2011). "Proton pump inhibitor side effects and drug interactions: much ado about nothing?". Cleve Clin J Med 78 (1): 39–49. doi:10.3949/ccjm.77a.10087. PMID 21199906.
- ^ Yang YX, Lewis JD, Epstein S, Metz DC (2006). "Long-term proton pump inhibitor therapy and risk of hip fracture". JAMA 296 (24): 2947–2953. doi:10.1001/jama.296.24.2947. PMID 17190895.
- ^ Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD (August 2008). "Use of proton pump inhibitors and risk of osteoporosis-related fractures". CMAJ 179 (4): 319–326. doi:10.1503/cmaj.071330. PMC 2492962. PMID 18695179. http://www.cmaj.ca/cgi/content/full/179/4/319.
- ^ "Proton pump inhibitors and Clostridium difficile". Bandolier. 2003. http://www.medicine.ox.ac.uk/bandolier/booth/Pharmacy/PPIcdiff.html. Retrieved 2007-07-13.
- ^ Herzig SJ, Howell MD, Ngo LH, Marcantonio ER (May 2009). "Acid-suppressive medication use and the risk for hospital-acquired pneumonia". JAMA 301 (20): 2120–2128. doi:10.1001/jama.2009.722. PMID 19470989. http://jama.ama-assn.org/content/301/20/2120.full.pdf+html.
- ^ Tubulointerstitial Nephritis at Merck Manual of Diagnosis and Therapy Professional Edition
- ^ Ray S, Delaney M, Muller AF (2010). "Proton pump inhibitors and acute interstitial nephritis". BMJ 341: c4412–c4412. doi:10.1136/bmj.c4412. PMID 20861097. http://www.bmj.com/content/341/bmj.c4412.full.
- ^ Thomson AB, Sauve MD, Kassam N, Kamitakahara H (May 2010). "Safety of the long-term use of proton pump inhibitors". World J Gastroenterol 16 (19): 2323–30. doi:10.3748/wjg.v16.i19.2323. PMC 2874135. PMID 20480516. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2874135.
- ^ a b Stedman CA, Barclay ML (August 2000). "Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors". Aliment Pharmacol Ther 14 (8): 963–978. doi:10.1046/j.1365-2036.2000.00788.x. PMID 10930890.
- ^ Lau WC, Gurbel PA (March 2009). "The drug-drug interaction between proton pump inhibitors and clopidogrel". CMAJ 180 (7): 699–700. doi:10.1503/cmaj.090251. PMC 2659824. PMID 19332744. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2659824.
- ^ Norgard NB, Mathews KD, Wall GC (July 2009). "Drug-drug interaction between clopidogrel and the proton pump inhibitors". Ann Pharmacother 43 (7): 1266–1274. doi:10.1345/aph.1M051. PMID 19470853.
- ^ Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF (December 2001). "Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein". Naunyn Schmiedebergs Arch Pharmacol 364 (6): 551–557. doi:10.1007/s00210-001-0489-7. PMID 11770010.
- ^ Izzo, AA; Ernst, E (2009). "Interactions between herbal medicines and prescribed drugs: an updated systematic review". Drugs 69 (13): 1777–1798. doi:10.2165/11317010-000000000-00000. PMID 19719333.
- ^ "Omeprazole, in The Free Medical Dictionary". http://medical-dictionary.thefreedictionary.com/omeprazole. Retrieved 11 November 2010.
- ^ "Omeprazole". Drugs.com. http://www.drugs.com/monograph/omeprazole.html. Retrieved 11 November 2010.
- ^ "Zegird, How to take". rxlist.com. http://www.rxlist.com/zegerid-drug-patient.htm#howtake. Retrieved 11 November 2010.
- ^ essential drug information. MIMS USA. Retrieved 20 December 2009.[verification needed]
- ^ Cass, QB; Lima, VV; Oliveira, RV; Cassiano, NM; Degani, AL; Pedrazzoli, J (December 2003). "Enantiomeric determination of the plasma levels of omeprazole by direct plasma injection using high-performance liquid chromatography with achiral-chiral column-switching". Journal of Chromatography B 798 (2): 275–281. doi:10.1016/j.jchromb.2003.09.053. PMID 14643507.
- ^ Baselt RC, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1146–7. ISBN 978-0- 9626523-7-0.
- ^ Nexium Prescribing Information. AstraZeneca Pharmaceuticals.
- ^ Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T (Jun 2005). "Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies". Drug Metab Pharmacokinet 20 (3): 153–67. doi:10.2133/dmpk.20.153. PMID 15988117.
- ^ "Santarus Receives FDA Approval for Immediate-Release Omeprazole Tablet with Dual Buffers". Santarus.
- ^ "FDA Approves Zegerid OTC for Over-the-Counter Treatment of Frequent Heartburn". Merck.
- ^ a b Farley, D (July–August 1995). "Making it easier to read prescriptions". FDA Consum 29 (6): 25–7. PMID 10143448. http://www.thebody.com/content/art13913.html.
External links
AstraZeneca Products Anastrozole · Atenolol · Brompheniramine · Budesonide · Disufenton sodium · Esomeprazole · FluMist · Gefitinib · Goserelin · Isosorbide mononitrate · Motavizumab · Omeprazole · Palivizumab · Propofol · Rosuvastatin · Tamoxifen · Ticagrelor · Vandetanib · Ximelagatran · ZolmitriptanPredecessors and
acquired companiesPeople Categories:- AstraZeneca
- Benzimidazoles
- Equine medications
- Phenol ethers
- Proton pump inhibitors
- Pyridines
- Sulfoxides
- World Health Organization essential medicines
Wikimedia Foundation. 2010.