- Escitalopram
-
The Cipralex brand name should not be confused with the antibiotic drug ciprofloxacin or its various brand names such as Cipro.
Escitalopram 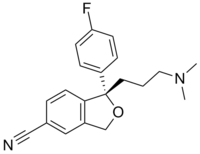

Systematic (IUPAC) name (S)-1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile Clinical data Trade names Lexapro AHFS/Drugs.com monograph MedlinePlus a603005 Pregnancy cat. C Legal status Rx Only (U.S) POM (U.K) Routes Oral Pharmacokinetic data Bioavailability 80% Protein binding ~56% Metabolism Liver, specifically the enzymes CYP3A4 and CYP2C19 Half-life 27–32 hours Identifiers CAS number 128196-01-0 
ATC code N06AB10 PubChem CID 146570 DrugBank DB01175 ChemSpider 129277 
UNII 4O4S742ANY 
ChEBI CHEBI:36791 
ChEMBL CHEMBL1508 
Chemical data Formula C20H21FN2O Mol. mass 324.392 g/mol
(414.43 as oxalate)SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Escitalopram (trade names Anxiset E(India) Lexapro, Cipralex, Seroplex, Lexamil, Lexam, Entact) is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with major depressive disorder and generalized anxiety disorder. Escitalopram is the S-stereoisomer (enantiomer) of the earlier Lundbeck drug citalopram, hence the name escitalopram. Escitalopram is noted for its high selectivity with serotonin reuptake inhibition. Its side effects are typical for the SSRI class. Only one independent study has shown that Escitalopram is more effective than citalopram, but in October 2011 it was reported that the company that sponsored the study had links to Lundbeck, the makers.[1] The similarity between Escitalopram and citalopram has led to accusations of 'evergreening', an accusation that Lundbeck has rejected.[1]
Contents
Medical uses
Escitalopram is primarily used for the treatment of major depressive disorder and general anxiety disorder in adults.[2] There is some evidence favouring escitalopram over the antidepressants citalopram and fluoxetine in the first two weeks of major depression.[3] Concerns of sponsorship bias with the studies is however noted.[3] In another review escitalopram and sertraline had the highest rate of efficacy and acceptability among adults receiving treatment for major depression with second-generation antidepressants.[4]
Adverse effects
The side effect profile of escitalopram is similar to that of other SSRIs. For example, according to the FDA analysis of depression trials common side effects for the highest approved dose of escitalopram are insomnia (14% vs. 4% for placebo), constricted pupils (15% vs. 5% for placebo), dry mouth (9% vs 3% for placebo), somnolence (9% vs 1% for placebo), dizziness (7% vs 2% for placebo), sweating (8% vs 1% for placebo), constipation (6% vs 1% for placebo), fatigue (6% vs 2% for placebo) and indigestion (6% vs. 1% for placebo).[5] Escitalopram, like other SSRIs, has been shown to affect sexual functions causing side effects such as decreased libido, delayed ejaculation, genital anesthesia,[6] and anorgasmia.[7][8] Although usually reversible upon discontinuation, these sexual side effects can last for months or years after the drug has been completely withdrawn.[9] This is known as Post SSRI Sexual Dysfunction. SSRI can rarely cause extrapyramidal side effects including akathisia through the indirect inhibition of dopamine.
Escitalopram is not associated with significant weight gain. For example, 0.6 kg mean weight change after 6 months of treatment with escitalopram for depression was insignificant and similar to that with placebo (0.2 kg).[10] 1.4–1.8 kg mean weight gain was reported in 8-month trials of escitalopram for depression,[11] and generalized anxiety disorder.[12] A 52-week trial of escitalopram for the long-term treatment of depression in elderly also found insignificant 0.6 kg mean weight gain.[13] Escitalopram may help reduce weight in those treated for binge eating associated obesity.[14]
An analysis conducted by the FDA found a statistically insignificant 1.5 to 2.4-fold (depending on the statistical technique used) increase of suicidality among the adults treated with escitalopram for psychiatric indications.[15][16][17] Similarly, the UK MHRA data indicate an 80% increase of suicide-related events, not reaching statistical significance, in the escitalopram vs placebo patients.[18] The authors of a related study note the general problem with statistical approaches: due to the rarity of suicidal events in clinical trials, it is hard to draw firm conclusions with a sample smaller than two million patients.[19] A single case report described a patient developing suicidal ideations after beginning treatment with escitalopram, and suicidal ideation disappearing after stopping the treatment.[20]
Escitalopram should be taken with caution when using St John's wort.[21] Exposure to escitalopram is increased moderately, by about 50%, when it is taken with omeprazole. The authors of this study, employed by Lundbeck, suggested that this increase is unlikely to be of clinical concern[22] Caution should be used when taking cough medicine containing dextromethorphan (DXM) as serotonin syndrome, liver damage, and other negative side effects have been reported.
Discontinuation symptoms
Main article: SSRI discontinuation syndromeEscitalopram discontinuation, particularly abruptly may cause certain withdrawal symptoms such as "electric shock" sensations (also known as "brain shivers" or "brain zaps"), dizziness, acute depressions and irritability,bladder control issues, as well as heightened senses of akathisia.[23]
Overdose
Excessive doses of escitalopram usually cause relatively minor untoward effects such as agitation and tachycardia. However, dyskinesia, hypertonia and clonus may occur in some cases. Plasma escitalopram concentrations are usually in a range of 20-80 μg/L in therapeutic situations and may reach 80-200 μg/L in the elderly, patients with hepatic dysfunction, those who are poor CYP2C19 metabolizers or following acute overdose. Monitoring of the drug in plasma or serum is generally accomplished using chromatographic methods. Chiral techniques are available to distinguish escitalopram from its racemate, citalopram.[24][25][26]
Pharmacology
Escitalopram increases intrasynaptic levels of the neurotransmitter serotonin by blocking the reuptake of the neurotransmitter into the presynaptic neuron. Of the SSRIs currently on the market escitalopram has the highest affinity for the human serotonin transporter (SERT). The enantiomer of escitalopram (R-citalopram) counteracts to a certain degree the serotonin-enhancing action of escitalopram. As a result, escitalopram is a more potent antidepressant than citalopram, which is a mixture of escitalopram and R-citalopram. In order to explain this phenomenon, researchers from Lundbeck proposed that escitalopram enhances its own binding via an additional interaction with another allosteric site on the transporter.[27] Further research by the same group showed that R-citalopram also enhances binding of escitalopram,[28] and therefore the allosteric interaction cannot explain the observed counteracting effect. In the most recent paper, however, the same authors again reversed their findings and reported that R-citalopram decreases binding of escitalopram to the transporter.[29] Although allosteric binding of escitalopram to the serotonin transporter is of unquestionable research interest, its clinical relevance is unclear since the binding of escitalopram to the allosteric site is at least 1000 times weaker than to the primary binding site.
In vitro studies using human liver microsomes indicated that CYP3A4 and CYP2C19 are the primary isozymes involved in the N-demethylation of escitalopram. The resulting metabolites, desmethylescitalopram and didesmethylescitalopram, are significantly less active and their contribution to the overall action of escitalopram is negligible.
History
Escitalopram was developed in close cooperation between Lundbeck and Forest Laboratories. Its development was initiated in the summer of 1997, and the resulting new drug application was submitted to the U.S. FDA in March 2001. The short time (3.5 years) it took to develop escitalopram can be attributed to the previous extensive experience of Lundbeck and Forest with citalopram, which has similar pharmacology.[30] The FDA issued the approval of escitalopram for major depression in August 2002 and for generalized anxiety disorder in December 2003. Escitalopram can be considered an example of "evergreening"[31] (also called "lifecycle management"[32])– the long-term strategy pharmaceutical companies use in order to extend the lifetime of a drug, in this case of the citalopram franchise. Escitalopram is an enantiopure compound of the racemic mixture citalopram, used for the same indication, and for that reason it required less investment and less time to develop. Two years after escitalopram's launch, when the patent on citalopram expired, the escitalopram sales successfully made up for the loss. On May 23, 2006, the FDA approved a generic version of escitalopram by Teva.[33] On July 14 of that year, however, the U.S. District Court of Delaware decided in favor of Lundbeck regarding the patent infringement dispute and ruled the patent on escitalopram valid.[34]
In 2006 Forest Laboratories was granted an 828 day (2 years and 3 months) extension on its US patent for escitalopram.[35] This pushed the patent expiry from December 7, 2009 to to September 14, 2011. Together with the 6-month pediatric exclusivity, the final expiration date is March 14, 2012.
Controversy
According to The New York Times, aggressive pharmaceutical marketing of escitalopram by Forest Laboratories has been controversial: the generic alternatives to the drug are cheaper, but a substantial number of doctors continue to prescribe the more expensive proprietary drug. The United States Senate Special Committee on Aging has released portions of the "Lexapro Fiscal 2004 Marketing Plan" which gives some of the details of the plans to promote use of the drug by doctors.[36]
In 2004, two separate civil suits alleging illegal marketing of citalopram and escitalopram for use by children and teenagers by Forest were initiated by two whistleblowers, one by a non-practicing physician named Joseph Piacentile, and the other by a Forest salesman named Christopher Gobble who was disturbed by what he witnessed at Forest.[37]
In February 2009, these two suits received support from the US Attorney for Massachusetts and were combined into one. Eleven states and the District of Columbia have also filed notices of intention to intervene as plaintiffs in the action. At the time, these drugs were approved only for use by adults and the application for use of citalopram in children was specifically rejected by the FDA. Although it is not illegal for physicians to prescribe a medicine for an off-label use not approved by the Food and Drug Administration, it is illegal for a manufacturer to promote the drugs for such uses. The government alleged that a research study showing lack of effectiveness when taken by children was concealed from its own medical advisers and sales personnel, as well as from researchers who conducted a study financed by the company. From 2001 to 2004, Forest heavily promoted results from another clinical trial it had financed which showed the drug was effective. Federal prosecutors also allege that the company has paid kickbacks to doctors to induce them to prescribe the medicines to children. The kickbacks allegedly included baseball tickets, a $1000 certificate to one of the most expensive New York restaurants, and paid vacations. Further, the complaint alleges that in September 2004, a Forest executive testified before Congress: “I want to emphasize that, because the FDA has not approved pediatric labeling of our products, Forest has always been scrupulous about not promoting the pediatric use of our antidepressant drugs, Celexa and Lexapro. That is the law and we follow it.” It is also alleged that the company conducted so-called "seeding studies" that were, in reality, marketing efforts to promote the drug's use by doctors.[38][39] Forest responded to these allegations that it "is committed to adhering to the highest ethical and legal standards, and off-label promotion and improper payments to medical providers have consistently been against Forest policy.[40]"
Only one independent study has shown that Escitalopram is more effective than citalopram, but in October 2011 it was reported that the Russian company that sponsored the study, Arbacom, had employee links to Lundbeck, the makers.[1] In Britain, the form Cipralex costs £14.91, while Lundbeck's older Cipramil can be found for £1.31. The Independent newspaper reported that this costs Britain's National Health Service (NHS) almost £25m extra per year, with no clear clinical benefits.[1] The Independent also describes the patenting of Escitalopram as an example of evergreening - slightly changing a drug that is about to go off-patent in order to acquire a patent for the new version, despite it containing similar ingredients to the previous version.[1] Lundbeck has denied that it has 'evergreened' Escitalopram.
References
- ^ a b c d e NHS pays millions of pounds more than it needs to for drugs, Independent. Retrieved 05/10/2011
- ^ "Escitalopram Oxalate". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/escitalopram-oxalate.html. Retrieved 3 April 2011.
- ^ a b Cipriani, A; Santilli, C, Furukawa, TA, Signoretti, A, Nakagawa, A, McGuire, H, Churchill, R, Barbui, C (2009 Apr 15). Cipriani, Andrea. ed. "Escitalopram versus other antidepressant agents for depression.". Cochrane database of systematic reviews (Online) (2): CD006532. doi:10.1002/14651858.CD006532.pub2. PMID 19370639.
- ^ Cipriani, A; Furukawa, TA, Salanti, G, Geddes, JR, Higgins, JP, Churchill, R, Watanabe, N, Nakagawa, A, Omori, IM, McGuire, H, Tansella, M, Barbui, C (2009 Feb 28). "Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis". Lancet 373 (9665): 746–58. doi:10.1016/S0140-6736(09)60046-5. PMID 19185342.
- ^ FDA Center for Drug Evaluation and Research (2001). "Review and evaluation of clinical data for application 21-323". http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-323.pdf_Lexapro_Medr_P1.pdf. Retrieved 2009-12-03.
- ^ Bolton JM, Sareen J, Reiss JP (2006). "Genital anesthesia persisting six years after sertraline discontinuation". J Sex Marital Ther 32 (4): 327–30. doi:10.1080/00926230600666410. PMID 16709553.
- ^ Clayton A, Keller A, McGarvey EL (2006). "Burden of phase-specific sexual dysfunction with SSRIs". Journal of Affective Disorders 91 (1): 27–32. doi:10.1016/j.jad.2005.12.007. PMID 16430968.
- ^ Lexapro prescribing information
- ^ Csoka AB, Bahrick AS, Mehtonen O-P (2008). "Persistent Sexual Dysfunction after Discontinuation of Selective Serotonin Reuptake Inhibitors (SSRIs)". J Sex Med. 5 (1): 227–33. doi:10.1111/j.1743-6109.2007.00630.x. PMID 18173768.
- ^ Baldwin DS, Reines EH, Guiton C, Weiller E (2007). "Escitalopram therapy for major depression and anxiety disorders". Ann Pharmacother 41 (10): 1583–92. doi:10.1345/aph.1K089. PMID 17848424.
- ^ Pigott TA, Prakash A, Arnold LM, Aaronson ST, Mallinckrodt CH, Wohlreich MM (2007). "Duloxetine versus escitalopram and placebo: an 8-month, double-blind trial in patients with major depressive disorder". Curr Med Res Opin 23 (6): 1303. doi:10.1185/030079907X188107. PMID 17559729.
- ^ Davidson JR, Bose A, Wang Q (2005). "Safety and efficacy of escitalopram in the long-term treatment of generalized anxiety disorder". J Clin Psychiatry 66 (11): 1441–6. doi:10.4088/JCP.v66n1115. PMID 16420082.
- ^ Kasper S, Lemming OM, de Swart H (2006). "Escitalopram in the long-term treatment of major depressive disorder in elderly patients". Neuropsychobiology 54 (3): 152–9. doi:10.1159/000098650. PMID 17230032.
- ^ Guerdjikova, Anna I.; Susan L. McElroy, Renu Kotwal, Jeffrey A. Welge, Erik Nelson, Katie Lake, David D' Alessio, Paul E. Keck Jr, James I. Hudson (2008). "High-dose escitalopram in the treatment of binge-eating disorder with obesity: a placebo-controlled monotherapy trial". Human Psychopharmacology: Clinical and Experimental 23 (1): 1–11. doi:10.1002/hup.899. PMID 18058852.
- ^ Levenson M, Holland C. "Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4272s1-04-FDA.ppt. Retrieved 2007-05-13.
- ^ Stone MB, Jones ML (2006-11-17). "Clinical Review: Relationship Between Antidepressant Drugs and Suicidality in Adults" (PDF). Overview for December 13 Meeting of Pharmacological Drugs Advisory Committee (PDAC). FDA. pp. 11–74. http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf. Retrieved 2007-09-22.
- ^ Levenson M, Holland C (2006-11-17). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Pharmacological Drugs Advisory Committee (PDAC). FDA. pp. 75–140. http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf. Retrieved 2007-09-22.
- ^ Gunnell D, Saperia J, Ashby D (2005). "Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomized controlled trials submitted to the MHRA's safety review". BMJ 330 (7488): 385. doi:10.1136/bmj.330.7488.385. PMC 549105. PMID 15718537. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=549105. Retrieved 2007-09-25.
- ^ Khan A, Schwartz K (2007). "Suicide risk and symptom reduction in patients assigned to placebo in duloxetine and escitalopram clinical trials: analysis of the FDA summary basis of approval reports". Ann Clin Psychiatry 19 (1): 31–6. doi:10.1080/10401230601163550. PMID 17453659.
- ^ Budur, Kumar; Hutzler, Jeffrey (June 2004). "Severe suicidal ideation with escitalopram (Lexapro): a case report". Primary Care Psychiatry 9 (2): 67–68. doi:10.1185/135525704125004222.
- ^ Karch, Amy (2006). 2006 Lippincott's Nursing Drug Guide. Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo: Lippincott Williams & Wilkins. ISBN 1-58255-436-6.
- ^ Malling, D.; Poulsen, M.; Søgaard, B. (2005). "The effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjects". British journal of clinical pharmacology 60 (3): 287–290. doi:10.1111/j.1365-2125.2005.02423.x. PMC 1884771. PMID 16120067. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1884771.
- ^ "Lexapro – Warnings". RxList. 12/08/2004. http://www.rxlist.com/cgi/generic/lexapro_wcp.htm. Retrieved 2006-10-22.
- ^ van Gorp F, Whyte IM, Isbister GK. Clinical and ECG effects of escitalopram overdose. Ann. Emer. Med. 54: 404-408, 2009.
- ^ Haupt D. Determination of citalopram enantiomers in human plasma by liquid chromatographic separation on a Chiral-AGP column. J. Chrom. B 685: 299-305, 1996.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 552-553.
- ^ For an overview of supporting data, see Sánchez C, Bøgesø KP, Ebert B, Reines EH, Braestrup C (2004). "Escitalopram versus citalopram: the surprising role of the R-enantiomer". Psychopharmacology (Berl.) 174 (2): 163–76. doi:10.1007/s00213-004-1865-z. PMID 15160261.
- ^ Chen F, Larsen MB, Sánchez C, Wiborg O (2005). "The S-enantiomer of R,S-citalopram, increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitors". European Neuropsychopharmacology 15 (2): 193–198. doi:10.1016/j.euroneuro.2004.08.008. PMID 15695064.
- ^ Mansari ME, Wiborg O, Mnie-Filali O, Benturquia N, Sánchez C, Haddjeri N (2007). "Allosteric modulation of the effect of escitalopram, paroxetine and fluoxetine: in-vitro and in-vivo studies". The International Journal of Neuropsychopharmacology 10 (1): 31–40. doi:10.1017/S1461145705006462. PMID 16448580.
- ^ "2000 Annual Report. p 28 and 33" (PDF). Lundbeck. 2000. http://www.materials.lundbeck.com/lundbeck/82/fullpdf/1.pdf. Retrieved 2007-04-07.
- ^ "New drugs from old. Presented at the Medical Journal Club, Morriston Hospital by Scott Pegler, Pharmacist at the National Health Service (UK) on November 20, 2006" (PPT). http://www.pharmedout.org/Pegler_New_Drugs_From_Old_Nov2006.ppt. Retrieved 2007-04-07.
- ^ "New drugs from old". Drug and Therapeutics Bulletin ;44:73-77; (BMJ Publishing Group Ltd.) 44 (10): 73–77. 2006. doi:10.1136/dtb.2006.441073. http://dtb.bmj.com/cgi/content/abstract/44/10/73?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=escitalopram&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT.
- ^ Miranda Hitti. "FDA OKs Generic Depression Drug – Generic Version of Lexapro Gets Green Light". WebMD. http://www.webmd.com/content/article/122/114778.htm. Retrieved 2007-10-10.
- ^ Marie-Eve Laforte (2006-07-14). "US court upholds Lexapro patent". FirstWord. http://www.firstwordplus.com/Fws.do?src=corp_site&articleid=7474B41ED0D14C20894E262219E24B62. Retrieved 2007-10-10.
- ^ "Forest Laboratories Receives Patent Term Extension for Lexapro" (Press release). PRNewswire-FirstCall. 2006-03-02. http://www.frx.com/news/PressRelease.aspx?ID=824655. Retrieved 2009-01-19.
- ^ Harris, "A Drug Maker’s Playbook Reveals a Marketing Strategy"
- ^ "Forest Laboratories: A Tale of Two Whistleblowers" article by Alison Frankel in The American Lawyer February 27, 2009
- ^ United States of America v. Forest Laboratories Full text of the federal complaint filed in the US District Court for the district of Massachusetts
- ^ "Drug Maker Is Accused of Fraud" article by Barry Meier and Benedict Carey in The New York Times February 25, 2009
- ^ "Forest Laboratories, Inc. Provides Statement in Response to Complaint Filed by U.S. Government" Forest press-release. February 26, 2009
- "A Drug Maker’s Playbook Reveals a Marketing Strategy" article in The New York Times by Gardiner Harris, September 1, 2009
Cited texts
- Royal Pharmaceutical Society of Great Britain (September 2009). British National Formulary (BNF 58). UK: BMJ Group and RPS Publishing. ISBN 9780853697787. http://www.bnf.org/bnf/.
External links
- Lexapro (Forest Laboratories) Official Lexapro Homepage
- Cipralex (Lundbeck) Official Cipralex Homepage
- Pharmacological information Lexapro
- Cipla Medpro Official Cipla Medpro Homepage
- portions of the "Lexapro Fiscal 2004 Marketing Plan" released by the United States Senate Special Committee on Aging
- U.S. National Library of Medicine: Drug Information Portal - Escitalopram
Antidepressants (N06A) Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) Alaproclate • Citalopram • Escitalopram • Femoxetine • Fluoxetine# • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • Seproxetine • Sertraline# • Vilazodone • Zimelidine‡Bicifadine • Clovoxamine • Desvenlafaxine • Duloxetine • Levomilnacipran • Eclanamine • Milnacipran • Sibutramine • VenlafaxineSerotonin–norepinephrine–dopamine reuptake inhibitors (SNDRIs)Brasofensine • BTS-74,398 • Cocaine • Diclofensine • DOV-21,947 • DOV-102,677 • DOV-216,303 • EXP-561 • Fezolamine • JNJ-7925476 • NS-2359 • PRC200-SS • Pridefine • SEP-225,289 • SEP-227,162 • TesofensineAmedalin • Atomoxetine/Tomoxetine • Binedaline • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • ViloxazineDopamine reuptake inhibitors (DRIs)Amineptine • Bupropion/Amfebutamone# • Cilobamine • Manifaxine • Methylphenidate • Nomifensine • Radafaxine • TametralineNorepinephrine-dopamine releasing agents (NDRAs)Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)4-Methyl-αMT • αET/Etryptamine • αMT/MetryptamineOthersIndeloxazine • Teniloxazine • Tramadol • ViqualineReceptor antagonists and/or reuptake inhibitors Serotonin antagonists and reuptake inhibitors (SARIs)Serotonin modulators and stimulators (SMSs)VortioxetineTricyclic and tetracyclic antidepressants (TCAs/TeCAs) TricyclicsAmezepine • Amineptine • Amitriptyline# • Amitriptylinoxide • Azepindole • Butriptyline • Cianopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Mariptiline • Melitracen • Metapramine • Mezepine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Pipofezine • Propizepine • Protriptyline • Quinupramine • Tampramine • Tianeptine • Tienopramine • Trimipramine;7-OH-Amoxapine • Amoxapine • Aptazapine • Azipramine • Ciclazindol • Ciclopramine • Esmirtazapine • Loxapine • Maprotiline • Mazindol • Mianserin • Mirtazapine • Oxaprotiline • Setiptiline/TeciptilineMonoamine oxidase inhibitors (MAOIs) NonselectiveIrreversible: Benmoxin • Echinopsidine • Iproclozide • Iproniazid • Isocarboxazid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Safrazine • Tranylcypromine; Reversible: Caroxazone • Paraxazone;MAOA-SelectiveIrreversible: Clorgiline; Reversible: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Esuperone • Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima;MAOB-SelectiveIrreversible: Ladostigil • Mofegiline • Pargyline • Rasagiline • Selegiline; Reversible: Lazabemide • MilacemideAzapirones and other 5-HT1A receptor agonists Alnespirone • Aripiprazole • Befiradol • Buspirone • Eptapirone • Flesinoxan • Flibanserin • Gepirone • Ipsapirone • Oxaflozane • Tandospirone • Vilazodone • ZalospironeSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersCategories:- Enantiopure drugs
- Nitriles
- Selective serotonin reuptake inhibitors
- Organofluorides
- Isobenzofurans
Wikimedia Foundation. 2010.