- Methylone
-
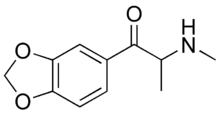
Methylone 
Systematic (IUPAC) name (±)-2-methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one Clinical data Pregnancy cat. ? Legal status Unscheduled (AU) ? (CA) ? (UK) Schedule I (US) I-P(Poland)[1] Routes Oral, Insufflation, Rectal Identifiers CAS number 186028-79-5  (racemic)
(racemic)
191916-41-3 (+)ATC code None PubChem CID 27281606 ChemSpider 21106350 
Chemical data Formula C11H13NO3 Mol. mass 207.23 g/mol SMILES eMolecules & PubChem Physical data Solubility in water 357 mg/mL (20 °C)  (what is this?) (verify)
(what is this?) (verify)Methylone, also known as "M1", 3,4-methylenedioxy-N-methylcathinone, bk-MDMA, is an entactogen and stimulant of the phenethylamine, amphetamine, and cathinone classes. It was originally patented by Nick Wald and Adam Fantoma in 1996 as an antidepressant.[2] The more intuitive abbreviation MDMC unfortunately can not be used for this chemical, since it had already been given to another earlier Shulgin creation, 3,4-ethylenedioxymethamphetamine. Methylone is a close structural analogue of MDMA, differing by the addition of a β-ketone group.[3]
Contents
Recreational use
At the end of 2004, a new designer drug called "Explosion" appeared in the Netherlands. This drug was sold as a liquid via the internet and in Dutch "smartshops" (known as "headshops" in the United States and some other countries), stores selling at the time non-scheduled, usually becoming illegal within a year of becoming well-known, psychoactive substances such as Salvia divinorum, Psilocybin mushrooms and other MDMA substitutes like BZP and TFMPP. The product is advertised as a "room odorizer" and is sold in plastic tubes containing 5 mL of liquid. The tubes cost between €10 and €15 ($13–$20) and do not present any information about the composition of Explosion; they contain only a label saying "Room odorizer Vanilla. Do not ingest" and "Keep away from children. Never use more than one bottle". Users have mentioned ingesting the liquid to reach euphoric stimulating effects similar to those of MDMA. The label circumvents Dutch regulations for illicit drugs and psychoactive substances when intentionally used for intoxication. Analysis of "Explosion" has confirmed that the active ingredient is methylone.[4]
Effects
Reported dosages range from 100 to 250 mg orally.[5] Some respondents say that increasing dose with methylone beyond 100–180 mg causes increased physical effects and does not substantially improve the empathic cognitive effects.[5]
The effects of methylone may include the following:
- Stimulation
- Euphoria or dysphoria, and anxiolysis or anxiogenesis, depending on the individual.
- An increase in sociability.
- Insomnia and restlessness
- Derealization/depersonalization, hallucinations, and psychosis, depending on the individual, and in all individuals with high dosage or extended use.
- Sympathomimetic
- Tachycardia and hypertension
- Hyperthermia and sweating
- Mydriasis and nystagmus
- Trismus and bruxism
- Anorexia
- Nausea and vomiting
Most of these effects are very similar to those of other psychostimulants.
Resemblance to MDMA
See also: Effects of MDMA on the human body Structural similarities between some amphetamine-like stimulants and their 3,4-methylenedioxy- derivatives.
Structural similarities between some amphetamine-like stimulants and their 3,4-methylenedioxy- derivatives.
Left: amphetamine, methamphetamine and methcathinone.
Right: MDA, MDMA, and methyloneMethylone resembles MDMA in its behavioural profile, as it substitutes for MDMA in rats trained to discriminate MDMA from saline. Methylone does not substitute for amphetamine or for the hallucinogenic DOM in animals trained to discriminate between these drugs and saline.[6] Further, also in common with MDMA, methylone acts on monoaminergic systems. In vitro, methylone has one third the potency of MDMA at inhibiting platelet serotonin accumulation and about the same in its inhibiting effects on the dopamine and noradrenaline transporters.[7][8][3]
In spite of these behavioural and pharmacological similarities between methylone and MDMA, the observed subjective effects of both drugs are not completely identical. Alexander Shulgin wrote of the former:[9]
"[Methylone] has almost the same potency of MDMA, but it does not produce the same effects. It has an almost antidepressant action, pleasant and positive, but not the unique magic of MDMA."
Pharmacology
Pharmacodynamics
Methylone acts as a mixed reuptake inhibitor/releasing agent of serotonin, norepinephrine, and dopamine.[3][10] In comparison to MDMA, it has approximately 3x lower affinity for the serotonin transporter, while its affinity for the norepinephrine and dopamine transporters is similar.[3][10] Notably, methylone's affinity for the vesicular monoamine transporter 2 (VMAT2) is about 13x lower than that of MDMA.[3] The results of these differences in pharmacology relative to MDMA are that methylone is less potent in terms of dose, has more balanced catecholaminergic effects relative to serotonergic, and behaves more like a reuptake inhibitor like methylphenidate than a releaser like amphetamine; however, methylone has relatively robust releasing capabilities,[10] perhaps due to its ability to phosphorylate the monoamine transporters being similar in potency relative to MDMA.[citation needed]
Pharmacokinetics
Little is currently known about the pharmacokinetics of methylone, aside from metabolism.
Metabolism
The two major metabolic pathways in mammals for methylone are N-demethylation to methylenedioxycathinone (MDC), and demethylation followed by O-methylation of the 3- or 4-hydroxy group to 4-hydroxy-3-methoxymethcathinone (HMMC) or 3-hydroxy-4-methoxymethcathinone (3-OH-4-MeO-MC). When 5 mg/kg of methylone was administered to rats, it was found that around 26% was excreted as HMMC within the first 48 hours (less than 3% excreted unchanged).[11]
Legal status
In the Netherlands, methylone is not yet scheduled as a drug of abuse, but is considered to be a psychoactive medicine. Because methylone is not registered officially, as such, it is forbidden to trade in methylone. The Minister of Health has asked the Coordination point Assessment and Monitoring new drugs group (CAM) to gather information about this substance, resulting possibly in an official risk assessment.[12] Until now, no research has been conducted on the toxicity of methylone, so nothing is known about the harmfulness of this new drug.
In New Zealand, although methylone is not explicitly scheduled and falls outside the strict definitions of an "amphetamine analogue" in the Misuse of Drugs Act, it is considered to be "substantially similar" to methcathinone and is thus considered by law enforcement authorities to be a Class C illegal drug. Methylone was sold in New Zealand for around 6 months from November 2005 to April 2006 as an MDMA substitute, under the name "Ease". The product was withdrawn after legal disputes with the government.[13][14]
In the UK, Methylone is illegal since the 16/04/2010 revision of the misuse of drugs act. Before this it was not specifically mentioned in United Kingdom (U.K.) law as the β-ketone was not covered under the Misuse of Drugs Act. In March 2010 plans were announced to make methylone and other cathinones, Class B drugs, "within weeks". While delayed by dissatisfaction in the ACMD, the revision was rushed through by the government with little regard for the views of the ACMD. The importation of the compounds was banned immediately.[15]
In Sweden, methylone was classed as a Harmful Substance since 2007, but since 21 September 2010 the drug was actively scheduled as a narcotic.[16] In Sweden the name in the books for the substance classified is 3,4-metylendioximetkatinon (Metylon).[17]
United States
- As of October 21, 2011 the DEA has issued an emergency ban on methylone. It is illegal to possess and distribute.
- Florida:
-
- In January 2011, it was reported that Florida Attorney General Pam Bondi issued an emergency ban on MDPV, Methylone, Mephedrone, 3-methoxymethcathinon, 3-fluoromethcathinone, and 4-fluoromethcathinone as media attention on products labeled as "bath salts" grew. These chemicals are now Schedule I under Florida law.
- Louisiana:
-
- In January 2011, the governor of Louisiana emergency scheduled 3,4-Methylenedioxymethcathinone (Methylone), 3,4-Methyenedioxypyrovalerone (MDPV), 4-Methylmethcathinone (Mephedrone), 4-methoxymethcathinone (Methedrone), 4-Fluoromethcathinone (Flephedrone), and 3-Fluoromethcathinone (3-FMC).
- Tennessee:
-
- On May 5, 2011, Tennessee Governor Bill Haslam signed a law making it a crime to knowingly produce, manufacture, distribute, sell, offer for sale or possess with intent produce, manufacture, distribute, sell, or offer for sale any product containing 3,4-Methylenedioxymethcathinone (Methylone), 3,4-Methyenedioxypyrovalerone (MDPV), 4-Methylmethcathinone (Mephedrone), 4-methoxymethcathinone (Methedrone), 4-Fluoromethcathinone (Flephedrone), and 3-Fluoromethcathinone (3-FMC).[18]
Etymology
"Methylone" is also a trademarked brand name for an injectable form of methylprednisolone, a corticosteroid hormone used to treat arthritis and severe allergic reactions; hence, methylone may be confused with it. Aside from context, they can be distinguished by the fact that the name will usually be capitalized when referring to the prescription drug.
A proposed alternate name is bk-MDMA, or beta-keto-MDMA. While this nomenclature has not caught on because the name "methylone" became widely used before the conflicting Methylone trademark was noticed, the analogous names for related chemicals bk-MDEA and bk-MBDB have become the established names for those substances.
See also
- Substituted cathinone
References
- ^ = WDU20111050614 "Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )". Internetowy System Aktów Prawnych. http://isap.sejm.gov.pl/DetailsServlet?id = WDU20111050614. Retrieved 17 June 2011.
- ^ WO 9639133
- ^ a b c d e Cozzi NV, Sievert MK, Shulgin AT, Jacob P, Ruoho AE (September 1999). "Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines". European Journal of Pharmacology 381 (1): 63–9. doi:10.1016/S0014-2999(99)00538-5. PMID 10528135. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(99)00538-5.
- ^ http://www.erowid.org/chemicals/methylone/methylone_info1.shtml
- ^ a b http://www.erowid.org/chemicals/methylone/methylone_dose.shtml.
- ^ TA Dal Cason, R Young, RA Glennon. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol. Biochem. Behav. 58, 1109–1116 (1997)
- ^ NV Cozzi, MK Sievert, AT Shulgin, P Jacob III, AE Ruoho. Methcathinone and 2 methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one (methylone) selectively inhibit plasma membrane catecholamine reuptake transporters. Soc. Neurosci. Abs., 24, 341.8 (1998).
- ^ NV Cozzi, AT Shulgin, AE Ruoho. Methcathinone (MCAT) and 2-methylamino-1-(3,4 methylenedioxyphenyl)propan-1-one (MDMCAT) inhibit [3H]serotonin uptake into human platelets. Amer. Chem. Soc. Div. Med. Chem. Abs., 215, 152 (1998).
- ^ "Cathinone | Ask Dr. Shulgin Online". http://www.cognitiveliberty.org/shulgin/adsarchive/cathinone.htm.
- ^ a b c Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology 559 (2–3): 132–7. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(06)01381-1.
- ^ Xenobioica. HT Kamata, N Shima, K Zaitsu, T Kamata, A Miki, M Nishikawa, M Katagi, H Tsuchihashi. (2006). Metabolism of methylone in humans and rats. Volume 36, Number 8 / August 2006.
- ^ van Amsterdam et al., 2004
- ^ "Party pill sparks official concern". One News. 7 April 2006. http://tvnz.co.nz/view/page/411424/697050. Retrieved 23 October 2011.
- ^ EASE trial terminated after conflicting advice. scoop.co.nz April 9, 2006
- ^ "Suspected mephedrone-type compound seized at airport". BBC News. 1 April 2010. http://news.bbc.co.uk/1/hi/uk/8599814.stm. Retrieved 3 April 2010.
- ^ [1], Förordning om ändring i förordningen (1992:1554) om kontroll av narkotika
- ^ "http://www.notisum.se/rnp/sls/lag/19990058.htm – Förordning (1999:58) om förbud mot vissa hälsofarliga varor". http://www.notisum.se/rnp/sls/lag/19990058.htm.
- ^ http://state.tn.us/sos/acts/107/pub/pc0169.pdf
External links
Entactogens Aminoindanes 5-IAI • ETAI • TAIAminotetralins 6-CATPhenethylamines
(and amphetamines,
cathinones, etc)4-CAB • 4-FA • 4-FMA • 4-MTA • 4-FPP • 5-APDB • 6-APDB • Ariadne • BDB • Brephedrone • Eutylone • Flephedrone • IAP • IMP • Metaescaline • Mephedrone • Methedrone • MMA • NAP • Norfenfluramine • Pentylone • PMA • PMEA • PMMA • TAPMDxx 2-Methyl-MDA • 5-Methyl-MDA • 6-Methyl-MDA • bk-MBDB (butylone) • bk-MDEA (ethylone) • bk-MDMA (methylone) • DMMDA • DMMDA-2 • EBDB • EBDP • EDMA • MDAI • MDMAI • MMAI • MDAT • MDMAT • MDA • MDDM • MDEA • MDIP • MDMA • MDMOH • MDMP • MDMPEA • MDOH • MDPEA • MDPH • MDPR • MMDPEA (Lophophine) • MBDB • MBDP • MMDA • MMDA-2 • MMDMATryptamines 4-Methyl-αET • αETStimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic aminesAdrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsDopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507Reuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersPhenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolCategories:- Amphetamine alkaloids
- Cathinones
- Entactogens and Empathogens
- Euphoriants
- Benzodioxoles
Wikimedia Foundation. 2010.