- Pramipexole
-
Pramipexole 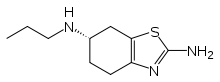
Systematic (IUPAC) name (S)-N6-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine Clinical data Trade names Mirapex AHFS/Drugs.com monograph MedlinePlus a697029 Pregnancy cat. B3(AU) C(US) Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Bioavailability >90% Protein binding 15% Half-life 8-12 hours Excretion Urine (90%), Feces (2%) Identifiers CAS number 104632-26-0 
ATC code N04BC05 PubChem CID 119570 DrugBank APRD00156 ChemSpider 106770 
UNII 83619PEU5T 
KEGG D05575 
ChEBI CHEBI:8356 
ChEMBL CHEMBL301265 
Chemical data Formula C10H17N3S Mol. mass 211.324 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Pramipexole (Mirapex, Mirapexin, Sifrol) is a non-ergoline dopamine agonist indicated for treating early-stage Parkinson's disease (PD) and restless legs syndrome (RLS).[1] It is also sometimes used off-label as a treatment for cluster headache and to counteract the problems with sexual dysfunction experienced by some users of the selective serotonin reuptake inhibitor (SSRI) antidepressants.[2] Pramipexole has shown robust effects on pilot studies in a placebo-controlled proof of concept study in bipolar disorder.[3] It is also being investigated for the treatment of clinical depression and fibromyalgia.[4][5][6]
Contents
Pharmacology
Pramipexole acts as a partial/full agonist at the following receptors:[7][8]
- D2S receptor (Ki = 3.9 nM; IA = 130%)
- D2L receptor (Ki = 2.2 nM; IA = 70%)
- D3 receptor (Ki = 0.5 nM; IA = 70%)
- D4 receptor (Ki = 5.1 nM; IA = 42%)
Pramipexole also possesses low/insignificant affinity (500-10,000 nM) for the 5-HT1A, 5-HT1B, 5-HT1D, and α2-adrenergic receptors.[7][9] It has negligible affinity (>10,000 nM) for the D1, D5, 5-HT2, α1-adrenergic, β-adrenergic, H1, and mACh receptors.[7][9] All sites assayed were done using human tissues.[7][8]
Parkinson's disease is a neurodegenerative disease affecting the substantia nigra, a component of the basal ganglia. The substantia nigra has a high quantity of dopaminergic neurons, which are nerve cells that release the neurotransmitter known as dopamine. When dopamine is released, it may activate dopamine receptors in the striatum, which is another component of the basal ganglia. When neurons of the substantia nigra deteriorate in Parkinson's disease, the striatum no longer properly receives dopamine signals. As a result, the basal ganglia can no longer regulate body movement effectively and motor function becomes impaired. By acting as an agonist for the D2, D3, and D4 dopamine receptors, pramipexole may directly stimulate the underfunctioning dopamine receptors in the striatum, thereby restoring the dopamine signals needed for proper functioning of the basal ganglia.
Side effects
Common side effects of pramipexole may include:[10][11]
- Headache
- Hyperalgesia (body aches and pains)
- Nausea and vomiting
- Sedation and somnolence
- Decreased appetite and subsequent weight loss
- Orthostatic hypotension (resulting in dizziness, lightheadedness, and possibly fainting, especially when standing up)
- Insomnia
- Hallucinations (seeing, hearing, smelling, tasting or feeling things that are not there)
- Twitching, twisting, or other unusual body movements
- Unusual tiredness or weakness
Several unusual adverse effects of pramipexole (and related D3-preferring dopamine agonist medications such as ropinirole) may include compulsive gambling, hypersexuality, and overeating,[12] even in patients without any prior history of these behaviours.[13] These behaviors have been reported to manifest in almost 14% of patients on DA agonist therapies. Other compulsive behaviors, such as excessive shopping and compulsive cross-dressing, have been reported.[14] L-DOPA is an indirect acting DA agonist with no specificity for any receptor subtypes. As it is the precursor for dopamine it is rarely associated with these disorders. These side effects are thought to be linked to the D3 activity of pramipexole, as D3 receptors are heavily expressed in brain regions involved in mood, behavior, and reward.[15]
Chemistry
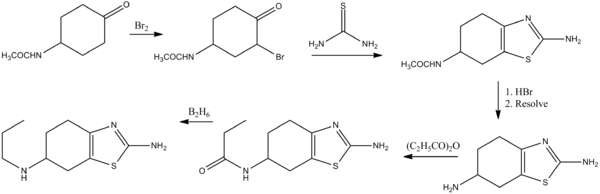
Pramiprexole can be synthesized from a cyclohexanone derivative by the following route:[16]
See also
- Dexpramipexole, the enantiomer of pramiproxole
- Piribedil
- Ropinirole
- Rotigotine
References
- ^ National Prescribing Service (2009). "Pramipexole for Parkinson's Disease". Medicines Update. Available at http://www.nps.org.au/consumers/publications/medicine_update/issues/Pramipexole_for_Parkinsons_disease
- ^ DeBattista C, Solvason HB, Breen JA, Schatzberg AF. (2000). "Pramipexole augmentation of a selective serotonin reuptake inhibitor in the treatment of depression.". J Clin Psychopharmacol. 20 (2): 274–275. doi:10.1097/00004714-200004000-00029. PMID 10770475.
- ^ Biol Psychiatry. 2004 Jul 1;56(1):54-60. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study.Zarate CA Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, Charney DS, Manji HK.PMID: 15219473
- ^ Lattanzi L, Dell'Osso L, Cassano P, Pini S, Rucci P, Houck PR, Gemignani A, Battistini G, Bassi A, Abelli M, Cassano GB. (2002). "Pramipexole in treatment-resistant depression: a 16-week naturalistic study.". Bipolar Disord. 4 (5): 307–314. doi:10.1034/j.1399-5618.2002.01171.x. PMID 12479663.
- ^ Cassano P, Lattanzi L, Soldani F, Navari S, Battistini G, Gemignani A, Cassano GB. (2004). "Pramipexole in treatment-resistant depression: an extended follow-up.". Depress Anxiety. 20 (3): 131–138. doi:10.1002/da.20038. PMID 15549689.
- ^ Holman AJ, Myers RR. (2005). "A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications.". Arthritis Rheum. 52 (8): 2495–2505. doi:10.1002/art.21191. PMID 16052595.
- ^ a b c d Kvernmo T, Härtter S, Burger E (August 2006). "A review of the receptor-binding and pharmacokinetic properties of dopamine agonists". Clinical Therapeutics 28 (8): 1065–78. doi:10.1016/j.clinthera.2006.08.004. PMID 16982285. http://linkinghub.elsevier.com/retrieve/pii/S0149-2918(06)00184-6.
- ^ a b Newman-Tancredi A, Cussac D, Audinot V, et al. (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor". The Journal of Pharmacology and Experimental Therapeutics 303 (2): 805–14. doi:10.1124/jpet.102.039875. PMID 12388667. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=12388667.
- ^ a b Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=12388666.
- ^ "MedlinePlus Drug Information: Pramipexole (Systemic)". United States National Library of Medicine. Archived from the original on 2006-09-26. http://web.archive.org/web/20060926023858/http://www.nlm.nih.gov/medlineplus/druginfo/uspdi/203739.html. Retrieved 2006-09-27.
- ^ "FDA Prescribing Information: Mirapex (pramipexole dihydrochloride)". Food and Drug Administration (United States). http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020667s014s017s018lbl.pdf. Retrieved 2008-12-31.
- ^ Wolters ECh, van der Werf YD, van den Heuvel OA. Parkinson's disease-related disorders in the impulsive-compulsive spectrum. Journal of Neurology. 2008 Sep;255 Suppl 5:48-56. PMID 18787882
- ^ Bostwick JM, Hecksel KA, Stevens SR, Bower JH, Ahlskog JE. Frequency of new-onset pathologic compulsive gambling or hypersexuality after drug treatment of idiopathic Parkinson disease. Mayo Clinic Proceedings. 2009 Apr;84(4):310-6. doi:10.4065/84.4.310 PMID 19339647
- ^ USA Today, Not Your Ordinary Side Effects, May 23, 2006
- ^ Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE (September 2005). "Pathological gambling caused by drugs used to treat Parkinson disease". Arch. Neurol. 62 (9): 1377–81. doi:10.1001/archneur.62.9.noc50009. PMID 16009751.
- ^ Schneider, Claus S.; Mierau, Joachim (1987). "Dopamine autoreceptor agonists: resolution and pharmacological activity of 2,6-diaminotetrahydrobenzothiazole and an aminothiazole analog of apomorphine". Journal of Medicinal Chemistry 30 (3): 494–8. doi:10.1021/jm00386a009. PMID 3820220.
External links
- Mirapex.com - Manufacturer's website
Antiparkinson agents (N04) Dopaminergics DA receptor agonistsAplindore • Apomorphine • Bromocriptine • Cabergoline • Ciladopa • Dihydroergocryptine • Lisuride • Pardoprunox • Pergolide • Piribedil • Pramipexole • Ropinirole • RotigotineAnticholinergics Benzatropine • Biperiden# • Bornaprine • Chlorphenoxamine • Cyrimine • Dexetimide • Dimenhydrinate • Diphenhydramine • Etanautine • Etybenzatropine • Mazaticol • Metixene • Orphenadrine • Phenglutarimide • Piroheptine • Procyclidine • Profenamine • Trihexyphenidyl • TropatepineOthers Urologicals, including antispasmodics (G04B) Acidifiers Urinary antispasmodics
(primarily antimuscarinics)Darifenacin • Emepronium • Fesoterodine • Flavoxate • Imidafenacin • Meladrazine • Oxybutynin • Propiverine • Solifenacin • Terodiline • Tolterodine • Trospium chlorideOther urologicals urea analogues: Acetohydroxamic acid • Salicylhydroxamic acid
other: Collagen • Dimethyl sulfoxide • Magnesium hydroxide • Pentosan polysulfate • Phenazopyridine • Phenyl salicylate • SuccinimideDopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugs Categories:- Dopamine agonists
- Thiazoles
Wikimedia Foundation. 2010.
