- Selegiline
-
Selegiline 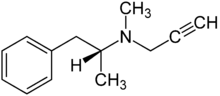
Systematic (IUPAC) name (R)-N-methyl-N-(1-phenylpropan-2-yl)prop-1-yn-3-amine Clinical data Trade names Eldepryl AHFS/Drugs.com monograph MedlinePlus a697046 Pregnancy cat. C (US) Legal status prescription only (unscheduled) (US) Routes Oral, transdermal, buccal Pharmacokinetic data Bioavailability 4.4% (oral, fasted), 20% (oral, after food), 18% (patch) Protein binding 90% Metabolism liver Half-life 1.5 hours (oral, single dose), 9 hours (oral, chronic) Excretion urine Identifiers CAS number 14611-51-9  [14611-52-0] (HCl)
[14611-52-0] (HCl)ATC code N04BD01 QN06AX90 PubChem CID 26757 DrugBank APRD00525 ChemSpider 24930 
UNII 2K1V7GP655 
KEGG D03731 
ChEBI CHEBI:9086 
ChEMBL CHEMBL972 
Chemical data Formula C13H17N Mol. mass 187.281 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Selegiline (L-deprenyl, Eldepryl, Emsam, Zelapar) is a drug used for the treatment of early-stage Parkinson's disease, depression and senile dementia. In normal clinical doses it is a selective irreversible MAO-B inhibitor, however in larger doses it loses its specificity and also inhibits MAO-A. Dietary restrictions are common for MAOI treatments, but special dietary restrictions for lower doses have been found to be unnecessary,[1] and dietary restrictions appear to be unnecessary at standard doses when selegiline is taken as Emsam, the transdermal patch form, as no adverse events due to diet have ever been reported with Emsam.[2] The drug was discovered by Jozsef Knoll et al. in Hungary. Selegiline belongs to a class of drugs called phenethylamines. Selegiline is a methamphetamine derivative with a propargyl group attached to the nitrogen atom.
Contents
History
Selegiline was discovered in Hungary in the 1960s. Joseph Knoll, a chair of pharmacology at the Semmelweis University in Budapest, was interested in the physiology of "drive" and the differences between high- and low-performing individuals. For his research, he required a molecule that combined amphetamine-like psychostimulant effect with a "psycho-energic" effect of monoamine oxidase inhibitors (MAOI). To do that, he decided to combine in the same molecule the structural features of the MAOI pargyline and the psychostimulant amphetamine. Knoll was a close friend of Meszaros, the research director of Chinoin, a Hungarian pharmaceutical company (later part of Sanofi). For this project, Meszaros put Knoll in contact with a chemist called Ecsery who worked in Chinoin in the field of phenethylamines. Escery made about 30 compounds, and Knoll selected the molecule of E-250 (deprenyl) based on its surprising properties. "The great discovery" (in Knoll's words) was that the new molecule did not increase blood pressure, unlike amphetamine, and moreover, it inhibited the blood pressure raising effect of amphetamine. The first publication on deprenyl in Hungarian appeared in 1964, followed by a paper in English in 1965. Deprenyl is a racemic compound, a mixture of two isomers called enantiomers. For the further pharmaceutical development, Knoll chose the (-)-enantiomer of deprenyl, which caused less hypermotility than the opposite (+)-enantiomer. This (-)-enantiomer (l-deprenyl, R-deprenyl) later has come to be called selegiline.[3]
In 1971, Knoll showed that selegiline selectively inhibits the B-isoform of monoamine oxidase (MAO-B) and proposed that it is unlikely to cause the infamous "cheese effect" (hypertensive crisis resulting from consuming foods containing tyramine) that plagues non-selective MAO inhibitors. A few years later, two Parkinson's researchers based in Vienna, Peter Riederer and Walther Birkmayer, realized that selegiline could be useful in Parkinson's disease. One of their colleagues, Moussa Youdim, visited Knoll in Budapest and took selegiline from him to Vienna. In 1975, the Birkmayer's group published the first paper on the effect of selegiline in Parkinson's disease.[3][4]
In 1967, a Hungarian psychiatrist Ervin Varga observed that racemic deprenyl given in large doses has an antidepressant action.[5] This study was largely forgotten until the 2000s when Sommerset Pharmaceuticals developed selegiline patch for depression.
Uses
The main use of selegiline is in the treatment of Parkinson's disease. It can be used on its own or in a combination with another agent, most often L-DOPA.[6] For the newly diagnosed Parkinson's patients, selegiline appears to slow the progression of the disease. It delays the time point when the L-DOPA (levodopa) treatment becomes necessary from 10-12 to 18 months.[7] The idea behind adding selegiline to levodopa is to decrease the dose of levodopa and thus reduce the motor complications of levodopa therapy.[8] Comparisons of patients on levodopa + placebo vs levodopa + selegiline showed that selegiline allowed reduction of the levodopa dose by about 40%. Selegiline + levodopa also extended the time until the levodopa dose had to be increased from 2.6 to 4.9 years.[7] As a result there were fewer motor complications in selegiline groups.[8] In one trial, selegiline + levodopa completely halted the progress of Parkinson's disease over 14 months, while in the placebo + levodopa group the deterioration of the patients' condition continued. However, the interpretation of this trial as proving neuroprotective action of selegiline has been questioned.[7]
As of February 28, 2006, selegiline has also been approved by the Food and Drug Administration (FDA) to treat major depression using a transdermal patch (Emsam Patch).[9] Selegiline (brand name Anipryl) is also used (at extremely high dosages relative to humans) in veterinary medicine to treat the symptoms of Cushing's disease and cognitive dysfunction (Canine Cognitive Dysfunction)[10][11] in dogs.[12][13][14] As of June 26, 2006, a selegiline transdermal patch is being tested for its effectiveness in treating ADHD.[15]
Several clinical studies are currently underway to evaluate selegiline's effectiveness in helping people stop smoking tobacco or cannabis.[16][17]
Side effects
Due to the primary metabolites of L-amphetamine and L-methamphetamine, selegiline shares many side effects seen with these sympathomimetic stimulants. Minor side effects such as dizziness, dry mouth, difficulty falling or staying asleep, muscle pain, rash, nausea and constipation have been seen. More serious side effects such as severe headache, tachycardia, arrhythmia, hallucinations, chorea, or difficulty breathing should be investigated by health professionals immediately.[18]
Pharmacology
Pharmacokinetics
Selegiline has a low oral bioavailability, which increases to moderate when ingested together with a high-fat meal (the molecule being liposoluble)[19].
Selegiline's oral bioavailability is drastically increased in females taking oral contraceptives (10- to 20-fold).[20] This could lead to loss of MAO-B selectivity in favor of an MAO-A selectivity, which in turn would make patients susceptible to the usual risks of unselective MAOIs such as tyramine-induced hypertensive crisis and serotonin toxicity when combined with serotonergics such as SSRIs.[20]
Mechanism of Action
Selegiline is a selective inhibitor of MAO-B; MAO-B metabolizes dopamine and phenylethylamine.[21] Selegiline exhibits little therapeutic benefit when used independently, but enhances and prolongs the anti-Parkinson effects of levodopa.[22]
Metabolites
Desmethylselegiline
Desmethylselegiline may have neuroprotective antiapoptotic properties. A large multicenter study suggests a decrease in the disease progression of parkinsonism but may have reflected other symptomatic response.[21] Desmethylselegiline is metabolized by CYP2C19.[23]
L-amphetamine and L-methamphetamine
Selegiline is partly metabolized to l-methamphetamine, one of the two enantiomers of methamphetamine in vivo.[24] A characteristic metabolic pattern was noted, exemplified by a ratio of l-methamphetamine to l-amphetamine of about 2.8.[25] These stereoisomers are considered significantly less psychoactive and have little abuse potential in contrast to their D-isomers.[26] The stimulatory effect on locomotor activity and dopamine synthesis may be contributed to by the action of l-methamphetamine, a once thought to be inactive metabolite.[27] This locomotor effect at therapeutic doses was not apparent in comparison to placebo, but both l-amphetamine and l-methamphetamine had positive effects on genetic expression for memory enhancement in rats and other animals.[28]
This metabolic action may cause persons taking prescribed selegiline to test positive for amphetamine and or methamphetamine on drug screening tests.
Legal issues
Possibly due to the structural similarity to illegal stimulants, selegiline has been classified as a controlled substance in Japan and thus can only be obtained with a prescription or special government license.
In E for Ecstasy[29] (a book examining the uses of the street drug Ecstasy in the UK) the writer, activist and Ecstasy advocate Nicholas Saunders highlighted test results showing that certain consignments of the drug also contained selegiline. Consignments of Ecstasy known as "Strawberry" contained what Saunders described as a "potentially dangerous combination of ketamine, ephedrine and selegiline," as did a consignment of "Sitting Duck" Ecstasy tablets.[30]
Selegiline is not a controlled substance in the US but a prescription is required to obtain it within the US.
Emsam
In February 2006 the US Food and Drug Administration approved Emsam (selegiline), the first transdermal patch for use in treating major depression. The once a day patch works by delivering selegiline through the skin and into the bloodstream. Emsam can be used without the dietary restrictions that are needed for all oral MAO inhibitors that are approved for treating major depression, although the FDA requires warnings concerning dietary restrictions for the 9 and 12 mg doses due to theoretical concerns not supported by any reports of adverse events.[2] It comes in three sizes that deliver 6, 9, or 12 mg of selegiline per 24 hours. The patch is a matrix containing three layers consisting of a backing, an adhesive drug layer, and a release liner that is placed against the skin. EMSAM was developed by Somerset Pharmaceuticals, Inc. In December 2004, Bristol-Myers Squibb and Somerset entered into an agreement that provides Bristol-Myers Squibb with distribution rights to market EMSAM after approval in the United States.
Zelapar
Zelapar is a transmucosal preparation for human administration of selegiline. The quickly-dissolving lozenge is placed between cheek and gum and the medication enters the bloodstream directly. Because hepatic first-pass metabolism is bypassed, the effective dose is lower than oral (swallowed) selegiline. GI side effects are reportedly reduced compared to oral (swallowed) selegiline. Zelapar is manufactured by Valeant Pharmaceuticals [2].
Chemistry
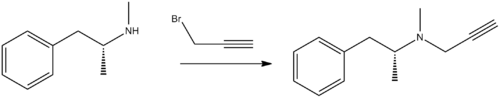
Selegiline, N-methyl-N-(2-propynyl)-2-methyl-1-phenylethyl-2-amine, is synthesized by the alkylation of (–)-methamphetamine using propargyl bromide.[31][32][33][34]
See also
References
- ^ Amsterdam, J. D. (2003-02). "A double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder". Journal of Clinical Psychiatry 64 (2): 208–14. doi:10.4088/JCP.v64n0216. PMID 12633131.
- ^ a b Cascade EF, Kalali AH (June 2007). "EMSAM: The First Year". Psychiatry 2007. http://www.psychiatrymmc.com/emsam-the-first-year/. Retrieved 2009-11-30.
- ^ a b Healy, David (2000). "The psychopharmacology of life and death. Interview with Joseph Knoll.". The Psychopharmacologists, Vol. III: Interviews. London: Arnold. pp. 81–110. ISBN 0-340-761105.
- ^ Birkmayer W, Riederer P, Youdim MB, Linauer W (1975). "The potentiation of the anti akinetic effect after L-dopa treatment by an inhibitor of MAO-B, Deprenil". J. Neural Transm. 36 (3–4): 303–26. doi:10.1007/BF01253131. PMID 1172524. http://link.springer.de/link/service/journals/00702/bibs/5036003/50360303.htm.
- ^ Varga E, Tringer L (1967). "Clinical trial of a new type promptly acting psychoenergetic agent (phenyl-isopropyl-methylpropinyl-HCl, "E-250")". Acta Med Acad Sci Hung 23 (3): 289–95. PMID 6056555.
- ^ Riederer P, Lachenmayer L, Laux G (August 2004). "Clinical applications of MAO-inhibitors". Curr. Med. Chem. 11 (15): 2033–43. PMID 15279566. http://www.bentham-direct.org/pages/content.php?CMC/2004/00000011/00000015/0007C.SGM.
- ^ a b c Riederer P, Lachenmayer L (November 2003). "Selegiline's neuroprotective capacity revisited". J Neural Transm 110 (11): 1273–8. doi:10.1007/s00702-003-0083-x. PMID 14628191.
- ^ a b Ives NJ, Stowe RL, Marro J et al. (September 2004). "Monoamine oxidase type B inhibitors in early Parkinson's disease: meta-analysis of 17 randomised trials involving 3525 patients". BMJ 329 (7466): 593. doi:10.1136/bmj.38184.606169.AE. PMC 516655. PMID 15310558. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=516655.
- ^ FDA Approves Emsam (Selegiline) as First Drug Patch for Depression
- ^ Lundgren, Becky. "Canine Cognitive Dysfunction". Veterinary Partner. http://www.veterinarypartner.com/Content.plx?P=A&A=2549. Retrieved 8 April 2011.
- ^ "Cognitive Dysfunction Syndrome". Long Beach Animal Hospital. http://www.lbah.com/cds.htm. Retrieved 8 April 2011.
- ^ http://www.petplace.com/drug-library/selegiline-hcl-anipryl/page1.aspx
- ^ "Anipryl consumer information". Drugs.com Vet. http://www.drugs.com/vet/anipryl-tablets.html. Retrieved 3 April 2011.
- ^ Braddock JA, Church DB, Robertson ID, (2004). "Selegiline Treatment of Canine Pituitary-Dependent Hyperadrenocorticism". Australian Veterinary Journal. http://www.lloydinc.com/pdfs/Endocrinology/Vol14_issue3_2004.pdf. Retrieved 8 April 2011. (PDF)
- ^ http://www.selegiline.com/adhd.html
- ^ "Effectiveness of Selegiline in Treating Marijuana Dependent Individuals". ClinicalTrials.gov. National Institute on Drug Abuse. March 2005. http://clinicaltrials.gov/show/NCT00218517. Retrieved 2007-02-16.
- ^ "Usefulness of Selegiline as an Aid to Quit Smoking". ClinicalTrials.gov. National Institute on Drug Abuse. July 2004. http://clinicaltrials.gov/show/NCT00129311. Retrieved 2007-02-16.
- ^ http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=medmaster&part=a697046
- ^ Jeffrey S. Barrett, Peter Szego, Shashank Rohatagi, Richard J. Morales, Kimberly E. DeWitt, Gregory Rajewski and Joyce Ireland (1996). “Absorption and Presystemic Metabolism of Selegiline Hydrochloride at Different Regions in the Gastrointestinal Tract in Healthy Males.” PHARMACEUTICAL RESEARCH 13(10):1535-1540, DOI: 10.1023/A:1016035730754
- ^ a b Laine K, Anttila M, Helminen A, Karnani H, Huupponen R (March 1999). "Dose linearity study of selegiline pharmacokinetics after oral administration: evidence for strong drug interaction with female sex steroids". Br J Clin Pharmacol 47 (3): 249–54. doi:10.1046/j.1365-2125.1999.00891.x. PMC 2014223. PMID 10215747. http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0306-5251&date=1999&volume=47&issue=3&spage=249.
- ^ a b Katzung, Bertram G. Basic & Clinical Pharmacology. 9th Edition. 2004. page 453. Lange Medical Books - McGraw Hill Publishers.
- ^ Katzung. Page 453
- ^ http://www.blackwell-synergy.com/doi/abs/10.1034/j.1600-0773.2000.d01-38.x Selegiline Metabolism and Cytochrome P450 Enzymes
- ^ Engberg G, Elebring T, Nissbrandt H (1991). "Deprenyl (selegiline), a selective MAO-B inhibitor with active metabolites; effects on locomotor activity, dopaminergic neurotransmission and firing rate of nigral dopamine neurons". J. Pharmacol. Exp. Ther. 259 (2): 841–7. PMID 1658311.
- ^ www.astm.org/JOURNALS/FORENSIC/PAGES/2587.htm
- ^ Are metabolites of l-deprenyl (selegiline) useful ...[J Neural Transm Suppl. 1996] - PubMed Result
- ^ Deprenyl (selegiline), a selective MAO-B inhibitor with active metabolites; effects on locomotor activity, dopaminergic neurotransmission and firing rate of nigral dopamine neurons. Engberg G, Elebring T, Nissbrandt H.
- ^ The levo enantiomer of amphetamine increases memory consolidation and gene expression in the hippocampus without producing locomotor stimulation. Wiig KA, Whitlock JR, Epstein MH, Carpenter RL, Bear MF.
- ^ Saunders, N., & Heron, L., (1993) E for Ecstasy (Paperback), N. Saunders, London. (ISBN 0950162884)
- ^ See: [1] for details online.
- ^ J. Knoll, E. Sanfai, DE 1568277 (1966).
- ^ J. Hermann Nee Voeroes, Z. Ecsery, G. Sabo, L. Arvai, L. Nagi, O. Orban, E. Sanfai, U.S. Patent 4,564,706 (1986)
- ^ B. Brunova, M. Ferenc, EP 344675 (1989)
- ^ Fowler, Joanna S. (1977). "2-Methyl-3-butyn-2-ol as an acetylene precursor in the Mannich reaction. A new synthesis of suicide inactivators of monoamine oxidase". The Journal of Organic Chemistry 42 (15): 2637. doi:10.1021/jo00435a026. PMID 874623.
External links
- Deprenyl (Selegiline) Can Slow Parkinson's Disease Safely According to the British Medical Journal
- FDA Approves Emsam (Selegiline) as First Drug Patch for Depression
Antiparkinson agents (N04) Dopaminergics DA receptor agonistsAplindore • Apomorphine • Bromocriptine • Cabergoline • Ciladopa • Dihydroergocryptine • Lisuride • Pardoprunox • Pergolide • Piribedil • Pramipexole • Ropinirole • RotigotineAnticholinergics Benzatropine • Biperiden# • Bornaprine • Chlorphenoxamine • Cyrimine • Dexetimide • Dimenhydrinate • Diphenhydramine • Etanautine • Etybenzatropine • Mazaticol • Metixene • Orphenadrine • Phenglutarimide • Piroheptine • Procyclidine • Profenamine • Trihexyphenidyl • TropatepineOthers Antidepressants (N06A) Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) Alaproclate • Citalopram • Escitalopram • Femoxetine • Fluoxetine# • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • Seproxetine • Sertraline# • Vilazodone • Zimelidine‡Bicifadine • Clovoxamine • Desvenlafaxine • Duloxetine • Levomilnacipran • Eclanamine • Milnacipran • Sibutramine • VenlafaxineSerotonin–norepinephrine–dopamine reuptake inhibitors (SNDRIs)Brasofensine • BTS-74,398 • Cocaine • Diclofensine • DOV-21,947 • DOV-102,677 • DOV-216,303 • EXP-561 • Fezolamine • JNJ-7925476 • NS-2359 • PRC200-SS • Pridefine • SEP-225,289 • SEP-227,162 • TesofensineAmedalin • Atomoxetine/Tomoxetine • Binedaline • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • ViloxazineDopamine reuptake inhibitors (DRIs)Amineptine • Bupropion/Amfebutamone# • Cilobamine • Manifaxine • Methylphenidate • Nomifensine • Radafaxine • TametralineNorepinephrine-dopamine releasing agents (NDRAs)Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)4-Methyl-αMT • αET/Etryptamine • αMT/MetryptamineOthersIndeloxazine • Teniloxazine • Tramadol • ViqualineReceptor antagonists and/or reuptake inhibitors Serotonin antagonists and reuptake inhibitors (SARIs)Serotonin modulators and stimulators (SMSs)VortioxetineTricyclic and tetracyclic antidepressants (TCAs/TeCAs) TricyclicsAmezepine • Amineptine • Amitriptyline# • Amitriptylinoxide • Azepindole • Butriptyline • Cianopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Mariptiline • Melitracen • Metapramine • Mezepine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Pipofezine • Propizepine • Protriptyline • Quinupramine • Tampramine • Tianeptine • Tienopramine • Trimipramine;7-OH-Amoxapine • Amoxapine • Aptazapine • Azipramine • Ciclazindol • Ciclopramine • Esmirtazapine • Loxapine • Maprotiline • Mazindol • Mianserin • Mirtazapine • Oxaprotiline • Setiptiline/TeciptilineMonoamine oxidase inhibitors (MAOIs) NonselectiveIrreversible: Benmoxin • Echinopsidine • Iproclozide • Iproniazid • Isocarboxazid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Safrazine • Tranylcypromine; Reversible: Caroxazone • Paraxazone;MAOA-SelectiveIrreversible: Clorgiline; Reversible: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Esuperone • Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima;MAOB-SelectiveIrreversible: Ladostigil • Mofegiline • Pargyline • Rasagiline • Selegiline; Reversible: Lazabemide • MilacemideAzapirones and other 5-HT1A receptor agonists Alnespirone • Aripiprazole • Befiradol • Buspirone • Eptapirone • Flesinoxan • Flibanserin • Gepirone • Ipsapirone • Oxaflozane • Tandospirone • Vilazodone • ZalospironeStimulants (N06B) Adamantanes Adaphenoxate • Adapromine • Amantadine • Bromantane • Chlodantane • Gludantane • Memantine • Midantane
Adenosine antagonists 8-Chlorotheophylline • 8-Cyclopentyltheophylline • 8-Phenyltheophylline • Aminophylline • Caffeine • CGS-15943 • Dimethazan • Paraxanthine • SCH-58261 • Theobromine • TheophyllineAlkylamines Arylcyclohexylamines Benocyclidine • Dieticyclidine • Esketamine • Eticyclidine • Gacyclidine • Ketamine • Phencyclamine • Phencyclidine • Rolicyclidine • Tenocyclidine • Tiletamine
Benzazepines 6-Br-APB • SKF-77434 • SKF-81297 • SKF-82958
Cholinergics A-84543 • A-366,833 • ABT-202 • ABT-418 • AR-R17779 • Altinicline • Anabasine • Arecoline • Cotinine • Cytisine • Dianicline • Epibatidine • Epiboxidine • GTS-21 • Ispronicline • Nicotine • PHA-543,613 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • SIB-1553A • SSR-180,711 • TC-1698 • TC-1827 • TC-2216 • TC-5619 • Tebanicline • UB-165 • Varenicline • WAY-317,538
Convulsants Anatoxin-a • Bicuculline • DMCM • Flurothyl • Gabazine • Pentetrazol • Picrotoxin • Strychnine • Thujone
Eugeroics Adrafinil • Armodafinil • CRL-40941 • Modafinil
Oxazolines 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone
Phenethylamines 1-(4-Methylphenyl)-2-aminobutane • 1-Phenyl-2-(piperidin-1-yl)pentan-3-one • 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane • 2-Fluoroamphetamine • 2-Fluoromethamphetamine • 2-OH-PEA • 2-Phenyl-3-aminobutane • 2-Phenyl-3-methylaminobutane • 2,3-MDA • 3-Fluoroamphetamine • 3-Fluoroethamphetamine • 3-Fluoromethcathinone • 3-Methoxyamphetamine • 3-Methylamphetamine • 3,4-DMMC • 4-BMC • 4-Ethylamphetamine • 4-FA • 4-FMA • 4-MA • 4-MMA • 4-MTA • 6-FNE • Alfetamine • α-Ethylphenethylamine • Amfecloral • Amfepentorex • Amfepramone • Amidephrine • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Arbutamine • β-Methylphenethylamine • β-Phenylmethamphetamine • Benfluorex • Benzedrone • Benzphetamine • BDB (J) • BOH (Hydroxy-J) • BPAP • Buphedrone • Bupropion (Amfebutamone) • Butylone • Cathine • Cathinone • Chlorphentermine • Cinnamedrine • Clenbuterol • Clobenzorex • Cloforex • Clortermine • D-Deprenyl • Denopamine • Dimethoxyamphetamine • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Dobutamine • DOPA (Dextrodopa, Levodopa) • Dopamine • Dopexamine • Droxidopa • EBDB (Ethyl-J) • Ephedrine • Epinephrine (Adrenaline) • Epinine (Deoxyepinephrine) • Etafedrine • Ethcathinone (Ethylpropion) • Ethylamphetamine (Etilamfetamine) • Ethylnorepinephrine (Butanefrine) • Ethylone • Etilefrine • Famprofazone • Fenbutrazate • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenmetramide • Fenproporex • Flephedrone • Fludorex • Furfenorex • Gepefrine • HMMA • Hordenine • Ibopamine • IMP • Indanylamphetamine • Isoetarine • Isoethcathinone • Isoprenaline (Isoproterenol) • L-Deprenyl (Selegiline) • Lefetamine • Lisdexamfetamine • Lophophine (Homomyristicylamine) • Manifaxine • MBDB (Methyl-J; "Eden") • MDA (Tenamfetamine) • MDBU • MDEA ("Eve") • MDMA ("Ecstasy", "Adam") • MDMPEA (Homarylamine) • MDOH • MDPR • MDPEA (Homopiperonylamine) • Mefenorex • Mephedrone • Mephentermine • Metanephrine • Metaraminol • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methoxamine • Methoxyphenamine • MMA • Methcathinone (Methylpropion) • Methedrone • Methoxyphenamine • Methylone • MMDA • MMDMA • MMMA • Morazone • N-Benzyl-1-phenethylamine • N,N-Dimethylphenethylamine • Naphthylamphetamine • Nisoxetine • Norepinephrine (Noradrenaline) • Norfenefrine • Norfenfluramine • Normetanephrine • Octopamine • Orciprenaline • Ortetamine • Oxilofrine • Paredrine (Norpholedrine, Oxamphetamine, Mycadrine) • PBA • PCA • PHA • Pargyline • Pentorex (Phenpentermine) • Pentylone • Phendimetrazine • Phenmetrazine • Phenpromethamine • Phentermine • Phenylalanine • Phenylephrine (Neosynephrine) • Phenylpropanolamine • Pholedrine • PIA • PMA • PMEA • PMMA • PPAP • Prenylamine • Propylamphetamine • Pseudoephedrine • Radafaxine • Ropinirole • Salbutamol (Albuterol; Levosalbutamol) • Sibutramine • Synephrine (Oxedrine) • Theodrenaline • Tiflorex (Flutiorex) • Tranylcypromine • Tyramine • Tyrosine • Xamoterol • Xylopropamine • Zylofuramine
Piperazines Piperidines 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine • 1-(3,4-Dichlorophenyl)-1-(piperidin-2-yl)butane • 2-Benzylpiperidine • 2-Methyl-3-phenylpiperidine • 3,4-Dichloromethylphenidate • 4-Benzylpiperidine • 4-Methylmethylphenidate • Desoxypipradrol • Difemetorex • Diphenylpyraline • Ethylphenidate • Methylnaphthidate • Methylphenidate (Dexmethylphenidate) • N-Methyl-3β-propyl-4β-(4-chlorophenyl)piperidine • Nocaine • Phacetoperane • Pipradrol • SCH-5472
Pyrrolidines 2-Diphenylmethylpyrrolidine • α-PPP • α-PBP • α-PVP • Diphenylprolinol • MDPPP • MDPBP • MDPV • MPBP • MPHP • MPPP • MOPPP • Naphyrone • PEP • Prolintane • Pyrovalerone
Tropanes 3-CPMT • 3'-Chloro-3α-(diphenylmethoxy)tropane • 3-Pseudotropyl-4-fluorobenzoate • 4'-Fluorococaine • AHN-1055 • Altropane (IACFT) • Brasofensine • CFT (WIN 35,428) • β-CIT (RTI-55) • Cocaethylene • Cocaine • Dichloropane (RTI-111) • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Norcocaine • PIT • PTT • RTI-31 • RTI-32 • RTI-51 • RTI-105 • RTI-112 • RTI-113 • RTI-117 • RTI-120 • RTI-121 (IPCIT) • RTI-126 • RTI-150 • RTI-154 • RTI-171 • RTI-177 • RTI-183 • RTI-193 • RTI-194 • RTI-199 • RTI-202 • RTI-204 • RTI-229 • RTI-241 • RTI-336 • RTI-354 • RTI-371 • RTI-386 • Salicylmethylecgonine • Tesofensine • Troparil (β-CPT, WIN 35,065-2) • Tropoxane • WF-23 • WF-33 • WF-60
Others 1-(Thiophen-2-yl)-2-aminopropane • 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-MDP • 2-Phenylcyclohexylamine • 2-Phenyl-3,6-dimethylmorpholine • 3-Benzhydrylmorpholine • 3,3-Diphenylcyclobutanamine • 5-(2-Aminopropyl)indole • 5-Iodo-2-aminoindane • AL-1095 • Amfonelic acid • Amineptine • Amiphenazole • Atipamezole • Atomoxetine (Tomoxetine) • Bemegride • Benzydamine • BTQ • BTS 74,398 • Carphedon • Ciclazindol • Cilobamine • Clofenciclan • Cropropamide • Crotetamide • Cypenamine • D-161 • Diclofensine • Dimethocaine • Efaroxan • Etamivan • EXP-561 • Fencamfamine • Fenpentadiol • Feprosidnine • G-130 • Gamfexine • Gilutensin • GSK1360707F • GYKI-52895 • Hexacyclonate • Idazoxan • Indanorex • Indatraline • JNJ-7925476 • JZ-IV-10 • Lazabemide • Leptacline • Levopropylhexedrine • Lomevactone • LR-5182 • Mazindol • Meclofenoxate • Medifoxamine • Mefexamide • Mesocarb • Methastyridone • Methiopropamine • N-Methyl-3-phenylnorbornan-2-amine • Nefopam • Nikethamide • Nomifensine • O-2172 • Oxaprotiline • Phthalimidopropiophenone • PNU-99,194 • Propylhexedrine • PRC200-SS • Rasagiline • Rauwolscine • Rubidium chloride • Setazindol • Tametraline • Tandamine • Trazium • UH-232 • Yohimbine
See also Sympathomimetic aminesNootropics (N06B) Acetylcholinesterases Ampakines CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • Sunifiram • UnifiramD1 Agonists Eugeroics GABAA α5 Inverse Agonists H3 Antagonists mACh Agonists Alvameline • Arecoline • Cevimeline • CI-1017 • Milameline • Sabcomeline • Talsaclidine • Tazomeline • XanomelinenACh Agonists AR-R17779 • Ispronicline • Nicotine • PNU-282,987 • SSR-180,711 • WAY-317,538Racetams Others Acetylcarnitine • Adafenoxate • Bifemelane • Bilobalide (Ginkgo Biloba) • Carbenoxolone • Cerlapirdine • Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine • Ensaculin • Fipexide • Idebenone • Indeloxazine • Latrepirdine • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • Pirisudanol • Pyritinol • S-17092 • Sulbutiamine • Taltirelin • Teniloxazine • Tricyanoaminopropene • VinpocetineAntioxidants Acetyl-L-Carnitine (ALCAR) • Alpha-Lipoic Acid (ALA) • Ascorbic Acid (Vitamin C) • Carotenoids (Vitamin A) • Curcumin • Edaravone • Polyphenols • Glutathione • Hydroxytyrosol • L-Carnitine • Ladostigil • Melatonin • N-Acetylcysteine (NAC) • N-Acetylserotonin (NAS) • Oleocanthal • Oleuropein • Rasagiline • Resveratrol • Selegiline • Selenium • Tocopherols (Vitamin E) • Tocotrienols (Vitamin E) • Tyrosol • Ubiquinone (Coenzyme Q) • Uric AcidDopaminergics Reuptake inhibitors PlasmalemmalDAT inhibitorsPiperazines: DBL-583 • GBR-12,935 • Nefazodone • Vanoxerine; Piperidines: BTCP • Desoxypipradrol • Dextromethylphenidate • Difemetorex • Ethylphenidate • Methylnaphthidate • Methylphenidate • Phencyclidine • Pipradrol; Pyrrolidines: Diphenylprolinol • Methylenedioxypyrovalerone (MDPV) • Naphyrone • Prolintane • Pyrovalerone; Tropanes: β-CPPIT • Altropane • Brasofensine • CFT • Cocaine • Dichloropane • Difluoropine • FE-β-CPPIT • FP-β-CPPIT • Ioflupane (123I) • Iometopane • RTI-112 • RTI-113 • RTI-121 • RTI-126 • RTI-150 • RTI-177 • RTI-229 • RTI-336 • Tenocyclidine • Tesofensine • Troparil • Tropoxane • WF-11 • WF-23 • WF-31 • WF-33; Others: Adrafinil • Armodafinil • Amfonelic acid • Amineptine • Benzatropine (Benztropine) • Bromantane • BTQ • BTS-74,398 • Bupropion (Amfebutamone) • Ciclazindol • Diclofensine • Dimethocaine • Diphenylpyraline • Dizocilpine • DOV-102,677 • DOV-21,947 • DOV-216,303 • Etybenzatropine (Ethylbenztropine) • EXP-561 • Fencamine • Fencamfamine • Fezolamine • GYKI-52,895 • Indatraline • Ketamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Mazindol • Medifoxamine • Mesocarb • Modafinil • Nefopam • Nomifensine • NS-2359 • O-2172 • Pridefrine • Propylamphetamine • Radafaxine • SEP-225,289 • SEP-227,162 • Sertraline • Sibutramine • Tametraline • Tedatioxetine • TripelennamineVMAT inhibitorsReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex (4-MAR, 4-MAX) • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-Hydroxyphenethylamine (2-OH-PEA) • 4-CAB • 4-Methylamphetamine (4-MA) • 4-Methylmethamphetamine (4-MMA) • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Methylphenethylamine (β-Me-PEA) • Benzodioxolylbutanamine (BDB) • Benzodioxolylhydroxybutanamine (BOH) • Benzphetamine • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethoxyamphetamine (DMA) • Dimethoxymethamphetamine (DMMA) • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • Ethcathinone (Ethylpropion) • Ethylamphetamine • Ethylbenzodioxolylbutanamine (EBDB) • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • Lophophine (Homomyristicylamine) • Mefenorex • Mephedrone • Methamphetamine (Desoxyephedrine, Methedrine; Dextromethamphetamine, Levomethamphetamine) • Methcathinone (Methylpropion) • Methedrone • Methoxymethylenedioxyamphetamine (MMDA) • Methoxymethylenedioxymethamphetamine (MMDMA) • Methylbenzodioxolylbutanamine (MBDB) • Methylenedioxyamphetamine (MDA, tenamfetamine) • Methylenedioxyethylamphetamine (MDEA) • Methylenedioxyhydroxyamphetamine (MDOH) • Methylenedioxymethamphetamine (MDMA) • Methylenedioxymethylphenethylamine (MDMPEA, homarylamine) • Methylenedioxyphenethylamine (MDPEA, homopiperonylamine) • Methylone • Ortetamine • Parabromoamphetamine (PBA) • Parachloroamphetamine (PCA) • Parafluoroamphetamine (PFA) • Parafluoromethamphetamine (PFMA) • Parahydroxyamphetamine (PHA) • Paraiodoamphetamine (PIA) • Paredrine (Norpholedrine, Oxamphetamine) • Phenethylamine (PEA) • Pholedrine • Phenpromethamine • Prenylamine • Propylamphetamine • Tiflorex (Flutiorex) • Tyramine (TRA) • Xylopropamine • Zylofuramine; Piperazines: 2,5-Dimethoxy-4-bromobenzylpiperazine (2C-B-BZP) • Benzylpiperazine (BZP) • Methoxyphenylpiperazine (MeOPP, paraperazine) • Methylbenzylpiperazine (MBZP) • Methylenedioxybenzylpiperazine (MDBZP, piperonylpiperazine); Others: 2-Amino-1,2-dihydronaphthalene (2-ADN) • 2-Aminoindane (2-AI) • 2-Aminotetralin (2-AT) • 4-Benzylpiperidine (4-BP) • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanylaminopropane (IAP) • Indanorex • Isometheptene • Methylhexanamine • Naphthylaminopropane (NAP) • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • Tuaminoheptane (Tuamine)Enzyme inhibitors PAH inhibitors3,4-DihydroxystyreneTH inhibitorsNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • L-Deprenyl (Selegiline) • Ladostigil • Lazabemide • Milacemide • Pargyline • Rasagiline • SafinamideDBH inhibitorsOthers L-Phenylalanine → L-Tyrosine → L-DOPA (Levodopa)Ferrous iron (Fe2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: Benzofuranylpropylaminopentane (BPAP) • Phenylpropylaminopentane (PPAP); Toxins: Oxidopamine (6-Hydroxydopamine)List of dopaminergic drugsPhenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolCategories:- Nootropics
- Monoamine oxidase inhibitors
- Antiparkinsonian agents
- Amphetamines
- Alkynes
- Enantiopure drugs
Wikimedia Foundation. 2010.