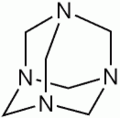
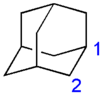- Adamantane
-
Adamantane 
 Adamantane[1]Other namesTricyclo[3.3.1.13,7]decane
Adamantane[1]Other namesTricyclo[3.3.1.13,7]decaneIdentifiers CAS number 281-23-2 
PubChem 9238 ChemSpider 8883 
ChEBI CHEBI:40519 
ChEMBL CHEMBL1614830 
Jmol-3D images Image 1
Image 2- C1C3CC2CC(CC1C2)C3
C1C2CC3CC1CC(C2)C3
Properties Molecular formula C10H16 Molar mass 136.23 g mol−1 Appearance White to off-white powder Density 1.08 g/cm3 (20 °C),[2] solid Melting point 270 °C (543 K)
Boiling point Sublimes
Solubility in water Poorly soluble Solubility in other solvents Soluble in hydrocarbons Refractive index (nD) 1.568[3] Structure Crystal structure cubic, space group Fm3m Coordination
geometry4 Dipole moment 0 D Hazards S-phrases 24/25/28/37/45 Main hazards Flammable Related compounds Related compounds: Memantine
Rimantadine
Amantadine (verify) (what is:
(verify) (what is:  /
/ ?)
?)
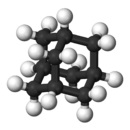
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free. Adamantane is the most stable among all the isomers with formula C10H16, which include the somewhat similar twistane. The spatial arrangement of carbon atoms is the same in adamantane molecule and in the diamond crystal. This motivates the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond).[4]
The discovery of adamantane in petroleum in 1933 launched a new chemistry field studying the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials and thermally stable lubricants.
Contents
History and synthesis
The possibility of the existence of a hydrocarbon with the C10H16 formula and diamond-like structure of the molecule was suggested by H. Decker at a conference in 1924. Dekker called this molecule decaterpene and was surprised that it had not been synthesized yet.[5]
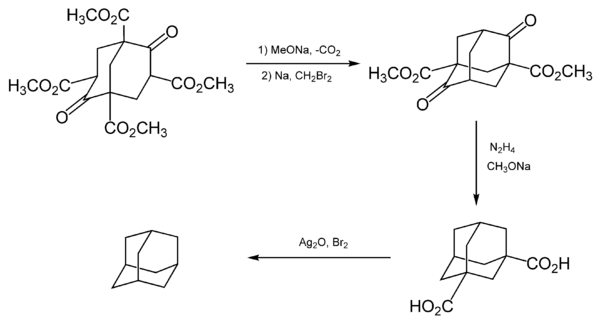
The first attempt of laboratory synthesis was made by German chemist Hans Meerwein in 1924 using reaction of formaldehyde with diethyl malonate in the presence of piperidine. Instead of adamantane, Meerwein obtained 1,3,5,7-tetracarbomethoxybicyclo[3.3.1]nonane-2,6-dione. This compound was later named Meerwein's ester and used in the syntheses of adamantane and its derivatives.[6] Later, another German chemist D. Bottger tried to obtain adamantane using Meerwein's ester as precursor. However, the product, tricyclo-[3.3.1.13,7] decane ring system, was again an adamantane derivative.[7]
Other researchers attempted to synthesize adamantane using phloroglucinol and derivatives of cyclohexanone but also without success.[8]
Adamantane was first synthesized by Vladimir Prelog in 1941 from Meerwein's ester.[9][10] The process was impractical as it contained five stages (simplified in the image below) and had a yield of about 0.16%. However, it was sometimes used to synthesize certain derivatives of adamantane.[8]
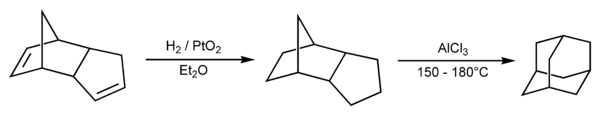
Prelog's method was refined in 1956. The decarboxylation yield was increased by the addition of the Heinsdecker pathway (11%), and the Hoffman reaction (24%) that raised the total yield to 6.5%.[11][12] The process was still too complex, and a more convenient method was accidentally found by Paul von Ragué Schleyer in 1957: dicyclopentadiene was first hydrogenated in the presence of a catalyst (e.g. platinum dioxide) and then transformed into adamantane using a Lewis acid (e.g. aluminium chloride) as another catalyst. This method increased the yield to 30–40% and provided an affordable source of adamantane; it therefore stimulated characterization of adamantane and is still used in the laboratory practice.[13][14] The adamantane synthesis yield was later increased to 60%[15] and nowadays, adamantane is an affordable chemical compound with a cost of the order $1/gram.
All the above methods yield adamantane in the form of polycrystalline powder. Using this powder, single crystals can be grown from the melt, solution or vapor phase (e.g. with the Bridgman–Stockbarger technique). Melt growth result in the worst crystalline quality with a mosaic spread in the X-ray reflection of about 1°. Best crystals are obtained from the liquid phase, but the growth is inpracticably slow – several months for a 5–10 mm crystal. Growth from the vapor phase is a reasonable compromise in terms of speed and quality.[2] Adamantane is sublimated in a quartz tube placed in a furnace, which is equipped with several heaters maintaining a certain temperature gradient (about 10 °C/cm for adamantane) along the tube. Crystallization starts at one end of the tube which is kept near the freezing point of adamantane. Slow cooling of the tube, while maintaining the temperature gradient, gradually shifts the melting zone (rate ~2 mm/hour) producing a single-crystal boule.[16]
Natural occurrence
Before adamantane was synthesized, it was isolated from petroleum by Czech chemists S. Landa and V. Machacek in 1933. They used fractional distillation where a sample of petroleum was gradually heated until leaving only solid impurities. The resulting steam enters a fractional distillation column. The temperature of the column decreases as the steam rises through the column. Therefore, hydrocarbon fractions condense along the column corresponding to their boiling point. Landa and Machacek could produce only a few milligrams of adamantane but noticed its high boiling and melting points. Because of the (assumed) similarity of its structure to that of diamond, the new compound was named adamantane.[8][17]
Petroleum remains the only natural source of adamantane; the content varies between 0.0001 and 0.03% depending on the oil field and is too low for commercial production.[18][19]
Beside adamantane, petroleum contains more than thirty of its derivatives.[18] Their isolation from a complex mixture of hydrocarbons is possible due to their high melting point and the ability to distill with water vapor and form stable adducts with thiourea.
Physical properties
Pure adamantane is a colorless crystalline solid with a characteristic camphor smell. It is practically insoluble in water, but readily soluble in nonpolar organic solvents.[20] Adamantane has an unusually high melting point for a hydrocarbon. At 270 °C, its melting point is much higher than other hydrocarbons with the same molecular weight, such as camphene (45 °C), limonene (–74 °C), ocimene (50 °C), terpinene (60 °C) or twistane (164 °C), or than a linear C10H22 hydrocarbon decane (–28 °C). However, adamantane slowly sublimates even at room temperature.[21] Adamantane can distill with water vapor.[19]
Structure
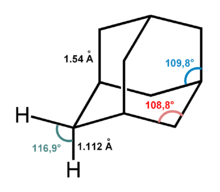
Adamantane molecule consists of three condensed cyclohexane rings fused in the chair conformation. The molecular parameters were deduced by electron diffraction and X-ray crystallography. The carbon-carbon bond length is 1.54 Å and is almost identical to that of diamond, and the carbon-hydrogen distance is 1.112 Å.[3]
At ambient conditions, adamantane crystallizes in a face-centered cubic structure (space group Fm3m, a = 9.426 ± 0.008 Å, four molecules in the unit cell) containing orientationally disordered adamantane molecules. This structure transforms into an ordered body-centered tetragonal phase (a = 6.641 Å, c = 8.875 Å) with two molecules per cell either upon cooling to 208 K or pressurizing to above 0.5 GPa.[8][21]
This phase transition is of the first order; it is accompanied by an anomaly in the heat capacity, elastic and other properties. In particular, whereas adamantane molecules freely rotate in the cubic phase, they are frozen in the tetragonal one; the density increases stepwise from 1.08 to 1.18 g/cm3 and the entropy changes by a significant amount of 1594 J/(mol·K).[2]
Hardness
Elastic constants of adamantane were measured using large (centimeter sized) single crystals and the ultrasonic echo technique. The principal value of the elasticity tensor, C11, was deduced as 7.52, 8.20 and 6.17 GPa for the <110>, <111> and <100> crystalline directions.[16] For comparison, the corresponding values for crystalline diamond are 1161, 1174 and 1123 GPa.[22] The arrangement of carbon atoms is the same in adamantane and diamond.[23] However, in the adamantane solid, molecules do not form a covalent lattice as in diamond, but interact through weak Van der Waals forces. As a result, adamantane crystals are very soft and plastic.[2][16][24]
Spectroscopy
The nuclear magnetic resonance (NMR) spectrum of adamantane consists of two poorly resolved signals, which correspond to the inequivalent sites 1 and 2 (see picture below). Their positions are 1.873 ppm and 1.756 ppm for adamantane in CDCl3 and 1H NMR, and are 28.46 ppm and 37.85 ppm for 13C NMR.[25] The simplicity of the NMR spectrum is a good monitor of the purity of adamantane – most derivatives have lower molecular symmetry and therefore more complex spectra.
Mass spectra of adamantane and its derivatives are rather characteristic. The main peak at m/z = 136 corresponds to the C10H+
16 ion. Its fragmentation results in weaker signals as m/z = 93, 80, 79, 67, 41 and 39.[3][25]The infrared absorption spectrum of adamantane is relatively simple because of the high symmetry of the molecule. The main absorption bands and their assignment are given in the table.[3]
Frequency of vibrations, cm−1 Assignment* 444 δ(CCC) 638 δ(CCC) 798 ν(C-C) 970 ρ(CH2), ν(C-C), δ(HCC) 1103 δ(HCC) 1312 ν(C-C), ω(CH2) 1356 δ(HCC), ω(CH2) 1458 δ(HCH) 2850 ν(C-H) in CH2 groups 2910 ν(C-H) in CH2 groups 2930 ν(C-H) in CH2 groups * Legends correspond to different types of oscillations: δ – deformation, ν – stretching, ρ and ω – out of plane deformation vibrations of CH2 groups. Optical activity
If adamantane molecules have four different substituents at every nodal carbon site then they are chiral and optically active. As in biphenyls, the center of chirality does not belong to any particular carbon atom.[26] Such optical activity was described in adamantane in 1969 with the four different substituents being hydrogen, bromine and methyl and carboxyl group. The values of specific rotation are small and are usually within 1°.[27][28]
Nomenclature
According to the rules of systematic nomenclature, adamantane should be called tricyclo[3.3.1.13,7]decane. However, IUPAC recommends using the name "adamantane".[1]
The adamantane molecule is composed of only carbon and hydrogen and has high Td symmetry. Therefore, its 16 hydrogen and 10 carbon atoms can be described by only two sites, which are labeled in the figure as 1 (4 equivalent sites) and 2 (6 equivalent sites).
The closest structural analogs of adamantane are noradamantane and homoadamantane, which respectively contain one less and one more CH2 link than the adamantane.
Chemical properties
Usually, hydrocarbons which contain only σ-bonds are relatively inert chemically. However, adamantane and its derivatives are highly reactive. This property is particularly evident in the ionic reactions where carbocations are formed as intermediates.
Adamantane cations
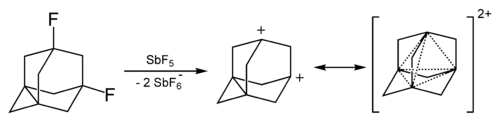
The adamantane cation can be produced by reacting 1-fluoro-adamantane with SbF5 and it has high stability compared with other tertiary carbocations.[29][30]
The dication of adamantane was obtained in solutions of superacids. It also has elevated stability due to the phenomenon called "three-dimensional aromaticity".[31]
Reactions
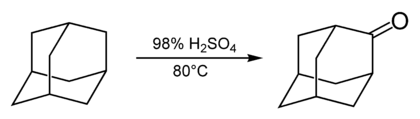
Most reactions of adamantane occur via the 3-coordinated carbon sites and are described in the subsections below. The 2-coordinated, bridging carbon sites are much less reactive. They are involved in the reaction of adamantane with concentrated sulfuric acid which produces adamantanone.[32]
The carbonyl group of adamantanone allows further reactions via the bridging site. For example, adamantanone is the starting compound for obtaining such derivatives of adamantane as 2-adamantanecarbonitrile[33] and 2-methyl-adamantane.[34]
Bromination
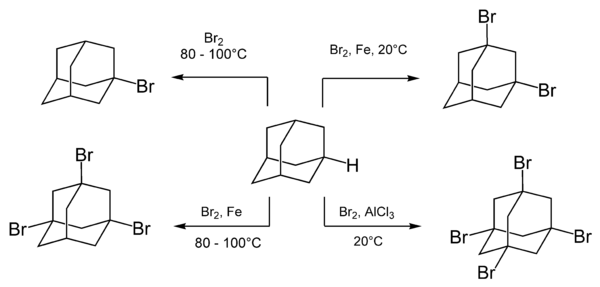
Adamantane readily reacts with various reagents brominating compounds such as molecular bromine. The composition and the ratio of the reaction products depend on the reaction conditions and especially presence of catalyst s.[18]
Boiling of adamantane with bromine results in a monosubstituted adamantane, 1-bromadamantane. Multiple substitution with bromine is achieved by adding a Lewis acid catalyst.[35]
The rate of bromination is accelerated upon addition of Lewis acids and is unchanged by irradiation or addition of free radicals. This indicates that the reaction occurs via an ionic mechanism.[8]
Fluorination
The first fluorinations of adamantane were conducted using 1-hydroxyadamantane[36] and 1-aminoadamantane as initial compounds. Later, fluorination was achieved starting from adamantane itself.[37] In all these cases, reaction proceeded via formation of adamantane cation which then interacted with fluorinated nucleophiles. Fluorination of adamantane with gaseous fluorine has also been reported.[38]
Carboxylation
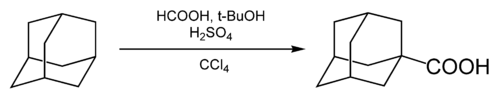
Carboxylation of adamantane was first reported in 1960, using formic acid as a carboxylating agent and carbon tetrachloride as a solvent.[39]
tert-butanol (t-BuOH) and sulfuric acid were added to generate adamantane cation; the cation was then carboxylated by carbon monoxide generated in situ in the interaction between the formic and sulfuric acids.[8] The fraction of carboxylated adamantane was 55-60%.[40]
Hydroxylation
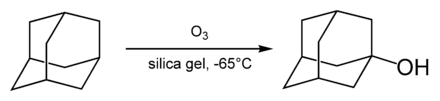
The simplest adamantane alcohol, 1-hydroxyadamantane, is readily formed by hydrolysis of 1-bromadamantane in aqueous solution of acetone. It can also be produced by ozonation of the adamantane:[41]
Others
Adamantane interacts with benzene in the presence of Lewis acids, resulting in a Friedel–Crafts reaction.[42] Aromatically substituted adamantane derivatives can be easily obtained starting from 1-hydroxyadamantane. In particular, the reaction with anisole proceeds under normal conditions and does not require a catalyst.[35]
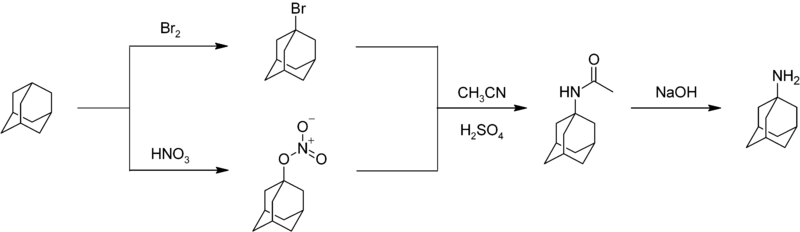
Nitration of adamantane is a difficult reaction characterized by moderate yields.[43] An important nitrogen-substituted drug amantadine can be prepared by reacting adamantane with bromine or nitric acid to give the bromide or nitroester at the 1- position. Reaction of either compound with acetonitrile affords the acetamide, which is hydrolyzed to give 1-adamantylamine:[44]
Uses
Adamantane itself enjoys few applications since it is merely an unfunctionalized hydrocarbon. It is used in some dry etching masks[45] and polymer formulations.
In solid-state NMR spectroscopy, adamantane is a common standard for chemical shift referencing.[46] In dye lasers, adamantane may be used to extend the life of the gain medium; it cannot be photoionized under atmosphere because its absorption bands lie in the vacuum-ultraviolet region of the spectrum. Photoionization energies have been determined for adamantane as well as for several bigger diamondoids.[47]
In medicine
All medical applications known so far involve not pure adamantane, but its derivatives. The first adamantane derivative used as a drug was amantadine – first (1967) as an antiviral drug against various strains of flu[48] and then to treat the Parkinson's disease.[49][50] Other drugs among adamantane derivatives include memantine, rimantadine, dopamantin, tromantadine, vildagliptin and karmantadin. Polymers of adamantane have been patented as antiviral agents against HIV.[51]
Potential technological applications
Some alkyl derivatives of adamantane have been used as a working fluid in hydraulic systems.[52] Adamantane-based polymers might find application for coatings of touchscreens,[53] and there are prospects for using adamantane and its homologues in nanotechnology. For example, the soft cage-like structure of adamantane solid allow incorporation of guest molecules, which can be released inside the human body upon breaking the matrix.[15][54]
Adamantane analogues
Many molecules adopt adamantane-like cage structures. Those include phosphorus trioxide P4O6, arsenic trioxide As4O6, phosphorus pentoxide P4O10 = (PO)4O6, phosphorus pentasulfide P4S10 = (PS)4S6, and hexamethylenetetramine C6N4H12 = N4(CH2)6.[55] Particularly notorious is tetramethylenedisulfotetramine, often shortened to "tetramine", a rodenticide banned in most countries for extreme toxicity to humans. The silicon analogue of adamantane, sila-adamantane, was synthesized in 2005.[56]
Adamantane cages can be stacked together to produce higher diamondoids, such as diamantane (C14H20 – two fused adamantane cages), triamantane (C18H24), tetramantane (C22H28), pentamantane (C26H32), hexamantane (C26H30), etc. Their synthesis is similar to that of adamantane and like adamantane, they can also be extracted from petroleum, though at even much smaller yields.
References
- ^ a b According to page 41 of a 2004 IUPAC guide, adamantane is the "preferred IUPAC name."
- ^ a b c d Windsor, C G; Saunderson, D H; Sherwood, J N; Taylor, D; Pawley, G S (1978). "Lattice dynamics of adamantane in the disordered phase". Journal of Physics C: Solid State Physics 11 (9): 1741. doi:10.1088/0022-3719/11/9/013.
- ^ a b c d Bagrii, E.I. (1989) (in Russian). Adamantanes: synthesis, properties, applications. Nauka. pp. 5–57. ISBN 5-02-001382-x. http://books.google.com/?id=8RA2AAAAIAAJ&dq=isbn=502001382X.
- ^ Alexander Senning. Elsevier's Dictionary of Chemoetymology. Elsevier, 2006, p. 6 ISBN 0444522395.
- ^ Decker H. (1924). "Versammlung deutscher Naturforscher und Ärzte. Innsbruck, 21–27 September 1924". Angew. Chem. 37 (41): 795. doi:10.1002/ange.19240374102.
- ^ Radcliffe, Marc D.; Gutierrez, Alberto; Blount, John F.; Mislow, Kurt (1984). "Structure of Meerwein's ester and of its benzene inclusion compound". Journal of the American Chemical Society 106 (3): 682. doi:10.1021/ja00315a037. http://www.oci.uzh.ch/efiles/OCVII/ja00315a037.pdf.
- ^ S. Coffey, S. Rodd (ed.) Chemistry of Carbon Compounds. Vol 2. Part C. Elsevier Publishing Co.: New York. 1969
- ^ a b c d e f Raymond C. Fort, Jr., And Paul Von R. Schleyers (1964). "Adamantane: Consequences of Diamondoid Structure". Chem. Rev. 64 (3): 277–300. doi:10.1021/cr60229a004.
- ^ Prelog V, Seiwerth R (1941). "Über die Synthese des Adamantans". Berichte 74 (10): 1644–1648. doi:10.1002/cber.19410741004.
- ^ Prelog V, Seiwerth R (1941). "Über eine neue, ergiebigere Darstellung des Adamantans". Berichte 74 (11): 1769–1772. doi:10.1002/cber.19410741109.
- ^ Stetter, H., Bander, O., and Neumann, W., Ber., 89, 1922 (1956).
- ^ McKervey, M (1980). "Synthetic approaches to large diamondoid hydrocarbons". Tetrahedron 36 (8): 971. doi:10.1016/0040-4020(80)80050-0.
- ^ Schleyer, P. von R. (1957). "A Simple Preparation of Adamantane". J. Am. Chem. Soc. 79 (12): 3292–3292. doi:10.1021/ja01569a086.
- ^ Schleyer, P. von R.; Donaldson, M. M.; Nicholas, R. D.; Cupas, C. (1973), "Adamantane", Org. Synth., http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv5p0016; Coll. Vol. 5: 16
- ^ a b G. Ali Mansoori (2007). Molecular building blocks for nanotechnology: from diamondoids to nanoscale materials and applications. Springer. pp. 48–55. ISBN 0387399372. http://books.google.com/?id=0-FtXsmeX8oC&pg=PA50.
- ^ a b c Drabble, J R; Husain, A H M (1980). "Elastic properties of adamantane single crystals". Journal of Physics C: Solid State Physics 13 (8): 1377. doi:10.1088/0022-3719/13/8/008.
- ^ Landa, S.; Machácek, V. (1933). Collection Czech. Chem. Commun. 5: 1.
- ^ a b c "Synthesis of adamantane" (in Russian). http://www.chem.msu.su/rus/teaching/oil/spezprakt-adam.html. Retrieved 2009-12-11.
- ^ a b Bagriy EI (1989). Adamantane: Synthesis, properties, application. Moscow: Nauka. pp. 58–123. ISBN 5-02-001382-x. http://books.google.com/?id=8RA2AAAAIAAJ&dq=isbn=502001382X.
- ^ "Adamantane" (in Russian). Encyclopedia of Chemistry. http://www.chemport.ru/chemical_encyclopedia_article_18.html. Retrieved 2009-12-11.
- ^ a b V Vijayakumar et al (2001). "Pressure induced phase transitions and equation of state of adamantane". J. Phys.: Condens. Matter 13 (9): 1961–1972. doi:10.1088/0953-8984/13/9/318.
- ^ Anastassakis, E.; Siakavellas, M. (1999). "Elastic and Lattice Dynamical Properties of Textured Diamond Films". Physica status solidi (b) 215: 189. doi:10.1002/(SICI)1521-3951(199909)215:1<189::AID-PSSB189>3.0.CO;2-X.
- ^ G. Ali Mansoori (2005). Principles of nanotechnology: molecular-based study of condensed matter in small systems. World Scientific. p. 12. ISBN 9812561544.
- ^ John Dalton Wright (1995). Molecular crystals. Cambridge University Press. p. 28. ISBN 0521477301. http://books.google.com/?id=7sroAgMASIEC&pg=PA28&dq=adamantane+soft&cd=12#v=onepage&q=adamantane%20soft.
- ^ a b NMR, IR and mass spectra of adamantane can be found in the SDBS database
- ^ J. March (1987). Organic chemistry. Reactions, mechanisms, structure. Advanced course for universities and higher education chemical. 1. M.: World. p. 137.
- ^ J. Applequist, P. Rivers, D. E. Applequist. (1969). "Theoretical and experimental studies of optically active bridgehead-substituted adamantanes and related compounds". J. Am. Chem. Soc. 91 (21): 5705–5711. doi:10.1021/ja01049a002.
- ^ H. Hamill, M. A. McKervey (1969). "The resolution of 3-methyl-5-bromoadamantanecarboxylic acid". Chem. Comm. (15): 864. doi:10.1039/C2969000864a.
- ^ Schleyer P. R., Fort R. C., Watts W. E. (1964). "Stable Carbonium Ions. VIII. The 1-Adamantyl Cation". J. Am. Chem. Soc. 86 (19): 4195–4197. doi:10.1021/ja01073a058.
- ^ George A. Olah, G. K. Surya Prakash, Joseph G. Shih, V. V. Krishnamurthy, Gheorge D. Mateescu, Gao Liang, Gyorgy Sipos, Volker Buss, Tamara M. Gund, Paul v. R. Schleyer. (1985). "Bridgehead adamantyl, diamantyl, and related cations and dications". J. Am. Chem. Soc. 107 (9): 2764–2772. doi:10.1021/ja00295a032.
- ^ W. Smith, Bochkov A., R. Caple (2001). Organic Synthesis. Science and art. M.: World. pp. 573. ISBN 5-03-003380-7. http://books.google.com/?id=EfNYAAAACAAJ&dq=isbn=5030033807.
- ^ H. W. Geluk and V. G. Keizer Adamantanone Organic Syntheses, Coll. Vol. 6, p. 48 (1988); Vol. 53, p. 8 (1973).
- ^ 2-Adamantanecarbonitrile Organic Syntheses, Coll. Vol. 6, p. 41 (1988); Vol. 57, p. 8 (1977).
- ^ Schleyer P. R., Nicholas R. D. (1961). "The Preparation and Reactivity of 2-Substituted Derivatives of Adamantane". J. Amer. Chem. Soc. 83 (1): 182–187. doi:10.1021/ja01462a036.
- ^ a b Nesmeyanov, A. N. (1969) (in Russian). Basic organic chemistry. p. 664.
- ^ Olah, George A.; Welch, John T.; Vankar, Yashwant D.; Nojima, Mosatomo; Kerekes, Istvan; Olah, Judith A. (1979). "Pyridinium poly (hydrogen fluoride): a convenient reagent for organic fluorination reactions". Journal of Organic Chemistry 44 (22): 3872–3881. doi:10.1021/jo01336a027.
- ^ Olah, George A.; Shih, Joseph G.; Singh, Brij P.; Gupta, B. G. B. (1983). "Ionic fluorination of adamantane, diamantane, and triphenylmethane with nitrosyl tetrafluoroborate/pyridine polyhydrogen fluoride (PPHF)". Journal of Organic Chemistry 48 (19): 3356–3358. doi:10.1021/jo00167a050.
- ^ Rozen, Shlomo.; Gal, Chava (1988). "Direct synthesis of fluoro-bicyclic compounds with fluorine". Journal of Organic Chemistry 53 (12): 2803–2807. doi:10.1021/jo00247a026.
- ^ H. Koch and W. Haaf, Angew. Chem., 72, 628 (1960).
- ^ 1-Adamantanecarboxylic acid Organic Syntheses, Coll. Vol. 5, p. 20 (1973); Vol. 44, p. 1 (1964).
- ^ Zvi Cohen, Haim Varkony, Ehud Keinan, and Yehuda Mazur Tertiary alcohols from hydrocarbons by ozonation on silica gel: 1-adamantanol Organic Syntheses, Coll. Vol. 6, p. 43 (1988); Vol. 59, p. 176 (1979)
- ^ Chalais, Stephane.; Corn lis, Andr; Gerstmans, Andr; Ko?odziejski, Wac?aw; Laszlo, Pierre; Mathy, Arthur; M tra, Pierre (1985). "Direct clay-catalyzed Friedel-Crafts arylation and chlorination of the hydrocarbon adamantane". Helvetica Chimica Acta 68 (5): 1196–1203. doi:10.1002/hlca.19850680516.
- ^ George W. Smith, Harry D. Williams. (1961). "Some Reactions of Adamantane and Adamantane Derivatives". J. Org. Chem. 26 (7): 2207–2212. doi:10.1021/jo01351a011.
- ^ I. K. Moiseev, R. I. Doroshenko and V. I. Ivanova (1976). "Synthesis of amantadine via the nitrate of 1-adamantanol". Pharmaceutical Chemistry Journal 10 (4): 450–451. doi:10.1007/BF00757832.
- ^ Watanabe, Keiji; et al. (2001). "Resist Composition and Pattern Forming Process". United States Patent Application 20010006752. Bandwidth Market, Ltd. http://www.bandwidthmarket.com/resources/patents/apps/. Retrieved 14 October 2005.[dead link]
- ^ Morcombe, Corey R.; Zilm, Kurt W. (2003). "Chemical Shift referencing in MAS solid state NMR". J. Magn. Reson. 162 (2): 479–486. doi:10.1016/S1090-7807(03)00082-X. PMID 12810033.
- ^ Lenzke, K. ; Landt, L.; Hoener, M. et al. (2007). "Experimental determination of the ionization potentials of the first five members of the nanodiamond series". J. Chem. Phys. 127 (8): 084320. doi:10.1063/1.2773725. PMID 17764261.
- ^ Maugh, T. (1979). "Panel urges wide use of antiviral drug". Science 206 (4422): 1058. doi:10.1126/science.386515. PMID 386515.
- ^ Lynn Sonnberg (2003). The Complete Pill Guide: Everything You Need to Know about Generic and Brand-Name Prescription Drugs. Barnes & Noble Publishing. p. 87. ISBN 0760742081. http://books.google.com/?id=TdcXatjup5cC&pg=PA87.
- ^ Blanpied TA, Clarke RJ, Johnson JW (2005). "Amantadine inhibits NMDA receptors by accelerating channel closure during channel block". Journal of Neuroscience 25 (13): 3312–22. doi:10.1523/JNEUROSCI.4262-04.2005. PMID 15800186. http://www.jneurosci.org/cgi/pmidlookup?view=long&pmid=15800186.
- ^ Boukrinskaia et al.. "Polymeric Adamantane Analogues" (U.S. Patent 5,880,154). http://www.google.com/patents/download/Polymeric_adamantane_analogues.pdf?id=WIcWAAAAEBAJ&output=pdf&sig=ACfU3U3Eid_gb9nV3HPEauQMBWHEyyJINg. Retrieved 2009-11-05.
- ^ "Adamantane" (in Russian). Krugosvet. http://www.krugosvet.ru/enc/nauka_i_tehnika/himiya/ADAMANTAN.html. Retrieved 2009-11-11.
- ^ H. Y. Jeong. (2002). "Synthesis and characterization of the first adamantane-based poly (p-phenylenevinylene) derivative: an intelligent plastic for smart electronic displays". Thin Solid Films 417 (1–2): 171–174. doi:10.1016/S0040-6090(02)00569-2.
- ^ Hamid Ramezani and G. Ali Mansoori. (2007). "Diamondoids as Molecular Building Blocks for Nanotechnology". Topics in Applied Physics 109 (Molecular Building Blocks for Nanotechnology.): 44–71. doi:10.1007/978-0-387-39938-6_4.
- ^ Vitall, J. J. (1996). "The Chemistry of Inorganic and Organometallic Compounds with Adamantane-Like Structures". Polyhedron 15 (10): 1585–1642. doi:10.1016/0277-5387(95)00340-1.
- ^ Jelena Fischer, Judith Baumgartner, Christoph Marschner. (2005). "Synthesis and Structure of Sila-Adamantane". Science 310 (5749): 825. doi:10.1126/science.1118981. PMID 16272116.
Categories:- Cycloalkanes
- Adamantanes
- C1C3CC2CC(CC1C2)C3
Wikimedia Foundation. 2010.