- Chemical shift
-
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule.[1][2][3] Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.
Some kinds atomic nuclei possess a magnetic moment (nuclear spin), which gives rise to different energy levels and resonance frequencies in a magnetic field. The total magnetic field experienced by a nucleus includes local magnetic fields induced by currents of electrons in the molecular orbitals (note that electrons have a magnetic moment themselves). The electron distribution of the same type of nucleus (e.g. 1H, 13C, 15N) usually varies according to the local geometry (binding partners, bond lengths, angles between bonds, ...), and with it the local magnetic field at each nucleus. This is reflected in the spin energy levels (and resonance frequencies). The variations of nuclear magnetic resonance frequencies of the same kind of nucleus, due to variations in the electron distribution, is called the chemical shift. The size of the chemical shift is given with respect to a reference frequency or reference sample (see also chemical shift referencing), usually a molecule with a barely distorted electron distribution.
Contents
Operating frequency
The operating (or Larmor) frequency ω0 of a magnet is calculated from the Larmor equation[4]
where B0 is the actual strength of the magnet in units like teslas or gauss, and γ is the gyromagnetic ratio of the nucleus being tested which is in turn calculated from its magnetic moment μ and spin number I with the nuclear magneton μN and the Planck constant h:
Thus, the proton operating frequency for a 1 T magnet is calculated as:
Chemical shift referencing
Chemical shift δ is usually expressed in parts per million (ppm) by frequency, because it is calculated from:
Since the numerator is usually in hertz, and the denominator in megahertz, delta is expressed in ppm.
The detected frequencies (in Hz) for 1H, 13C, and 29Si nuclei are usually referenced against TMS (tetramethylsilane) or DSS, which is assigned the chemical shift of zero. Other standard materials are used for setting the chemical shift for other nuclei.
Thus, an NMR signal that absorbs at 300 Hz higher than does TMS at an applied frequency of 300 MHz has a chemical shift of:
Although the frequency depends on the applied field the chemical shift is independent of it. On the other hand the resolution of NMR will increase with applied magnetic field resulting in ever increasing chemical shift changes.
The induced magnetic field
The electrons around a nucleus will circulate in a magnetic field and create a secondary induced magnetic field. This field opposes the applied field as stipulated by Lenz's law and atoms with higher induced fields (i.e., higher electron density) are therefore called shielded, relative to those with lower electron density. The chemical milieu of an atom can influence its electron density through the polar effect. Electron-donating alkyl groups, for example, lead to increased shielding while electron-withdrawing substituents such as nitro groups lead to deshielding of the nucleus. Not only substituents cause local induced fields. Bonding electrons can also lead to shielding and deshielding effects. A striking example of this are the pi bonds in benzene. Circular current through the hyperconjugated system causes a shielding effect at the molecule's center and a deshielding effect at its edges. Trends in chemical shift are explained based on the degree of shielding or deshielding.
Nuclei are found to resonate in a wide range to the left (or more rare to the right) of the internal standard. When a signal is found with a higher chemical shift:
- the applied effective magnetic field is lower, if the resonance frequency is fixed, (as in old traditional CW spectrometers)
- the frequency is higher, when the applied magnetic field is static, (normal case in FT spectrometers)
- the nucleus is more deshielded
- the signal or shift is downfield or at low field or paramagnetic
Conversely a lower chemical shift is called a diamagnetic shift, and is upfield and more shielded.
Diamagnetic shielding
In real molecules protons are surrounded by a cloud of charge due to adjacent bonds and atoms. In an applied magnetic field (B0) electrons circulate and produce an induced field (Bi) which opposes the applied field. The effective field at the nucleus will be B = B0 − Bi. The nucleus is said to be experiencing a diamagnetic shielding
Factors causing chemical shifts
Important factors influencing chemical shift are electron density, electronegativity of neighboring groups and anisotropic induced magnetic field effects.
Electron density shields a nucleus from the external field. For example in proton NMR the electron-poor tropylium ion has its protons downfield at 9.17 ppm, those of the electron-rich cyclooctatetraenyl anion move upfield to 6.75 ppm and its dianion even more upfield to 5.56 ppm.
A nucleus in the vicinity of an electronegative atom experiences reduced electron density and the nucleus is therefore deshielded. In proton NMR of methyl halides (CH3X) the chemical shift of the methyl protons increase in the order I < Br < Cl < F from 2.16 ppm to 4.26 ppm reflecting this trend. In carbon NMR the chemical shift of the carbon nuclei increase in the same order from around –10 ppm to 70 ppm. Also when the electronegative atom is removed further away the effect diminishes until it can be observed no longer.
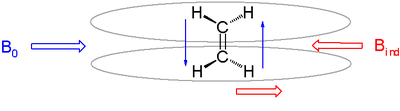
Anisotropic induced magnetic field effects are the result of a local induced magnetic field experienced by a nucleus resulting from circulating electrons that can either be paramagnetic when it is parallel to the applied field or diamagnetic when it is opposed to it. It is observed in alkenes where the double bond is oriented perpendicular to the external field with pi electrons likewise circulating at right angles. The induced magnetic field lines are parallel to the external field at the location of the alkene protons which therefore shift downfield to a 4.5 ppm to 7.5 ppm range. The three-dimensional space where a nucleus experiences diamagnetic shift is called the shielding zone with a cone-like shape aligned with the external field.
The protons in aromatic compounds are shifted downfield even further with a signal for benzene at 7.73 ppm as a consequence of a diamagnetic ring current.
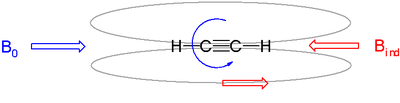
Alkyne protons by contrast resonate at high field in a 2–3 ppm range. For alkynes the most effective orientation is the external field in parallel with electrons circulation around the triple bond. In this way the acetylenic protons are located in the cone-shaped shielding zone hence the upfield shift.
Magnetic properties of most common nuclei
1H and 13C aren't the only nuclei susceptible to NMR experiments. A number of different nuclei can also be detected, although the use of such techniques is generally rare due to small relative sensitivities in NMR experiments (compared to 1H) of the nuclei in question, the other factor for rare use being their slender representation in nature/organic compounds.
Isotope Occurrence
in nature
(%)spin number l Magnetic moment μ[5] Electric quadrupole moment
(e×10−24 cm2)Operating frequency at 7 T
(MHz)Relative sensitivity 1H 99.984 1/2 2.79628 300.13 1 2H 0.016 1 0.85739 2.8 x 10−3 46.07 0.0964 10B 18.8 3 1.8005 7.4 x 10−2 32.25 0.0199 11B 81.2 3/2 2.6880 2.6 x 10−2 96.29 0.165 12C 98.9 0 13C 1.1 1/2 0.70220 75.47 0.0159 14N 99.64 1 0.40358 7.1 x 10−2 21.68 0.00101 15N 0.37 1/2 −0.28304 30.41 0.00104 16O 99.76 0 17O 0.0317 5/2 −1.8930 −4.0 x 10−3 40.69 0.0291 19F 100 1/2 2.6273 282.40 0.834 28Si 92.28 0 29Si 4.70 1/2 −0.55548 59.63 0.0785 31P 100 1/2 1.1205 121.49 0.0664 35Cl 75.4 3/2 0.92091 −7.9 x 10−2 29.41 0.0047 37Cl 24.6 3/2 0.68330 −6.2 x 10−2 24.48 0.0027 Magnetic properties of common nuclei[6] 1H, 13C, 15N, 19F and 31P are the five nuclei that have the greatest importance in NMR experiments:
- 1H because of high sensitivity and vast occurrence in organic compounds
- 13C because of being the key component of all organic compounds despite occurring at a low abundance (1.1%) compared to the major isotope of carbon 12C, which has a spin of 0 and therefore is NMR inactive.
- 15N because of being a key component of important biomolecules such as proteins and DNA
- 19F because of high relative sensitivity
- 31P because of frequent occurrence in organic compounds and moderate relative sensitivity
Other chemical shifts
The related Knight shift (first reported in 1949) is observed with pure metals. The NMR chemical shift in its present day meaning first appeared in journals in 1950. Chemical shifts with a different meaning appear in X-ray photoelectron spectroscopy as the shift in atomic core-level energy due to a specific chemical environment. The term is also used in Mössbauer spectroscopy, where similarly to NMR it refers to a shift in peak position due to the local chemical bonding environment. As is the case for NMR the chemical shift reflects the electron density at the atomic nucleus.[7]
See also
- MRI
- Protein NMR
- Solid-state NMR
- Zeeman effect
References
- ^ Spectrometric Identification of organic Compounds Silverstein, Bassler, Morrill 4th Ed. ISBN 047109700
- ^ Organic Spectroscopy William Kemp 3rd Ed. ISBN 0333417674
- ^ Basic 1H - 13C-NMR spectroscopy Metin Balei ISBN 0444518118
- ^ Chemical Shift | NMRCentral
- ^ In units of the nuclear magneton
- ^ CRC Handbook of Chemistry and Physics 65Th Ed
- ^ A Short History of Three Chemical Shifts Shin-ichi Nagaoka Vol. 84 No. 5 May 2007 Journal of Chemical Education 801
External links
- Online tutorials (these generally involve combined use of IR, 1H NMR, 13C NMR and mass spectrometry)
- Problem set 1, advanced (see also this link for more background information on spin-spin coupling)
- Problem set 2, moderate
- Problem set 4, moderate, German language (don't let that scare you away!)
- Problem set 5, the best!
- Combined solutions to problem set 5 (Problems 1–32) and (Problems 33–64)
Wikimedia Foundation. 2010.