- Magnetic moment
-
Electromagnetism 
Electricity · Magnetism Ampère's law · Electric current · Magnetic field · Magnetization · Magnetic flux · Biot–Savart law · Magnetic dipole moment · Gauss's law for magnetismLorentz force law · emf · Electromagnetic induction · Faraday’s law · Lenz's law · Displacement current · Maxwell's equations · EM field · Electromagnetic radiation · Liénard–Wiechert potential · Maxwell tensor · Eddy currentThe magnetic moment of a magnet is a quantity that determines the force that a magnet can exert on electric currents and the torque that a magnetic field will exert on it. A loop of electric current, a bar magnet, an electron, a molecule, and a planet all have magnetic moments.
Both the magnetic moment and magnetic field may be considered to be vectors having a magnitude and direction. The direction of the magnetic moment points from the south to north pole of a magnet. The magnetic field produced by a magnet is proportional to its magnetic moment as well. More precisely, the term magnetic moment normally refers to a system's magnetic dipole moment, which produces the first term in the multipole expansion of a general magnetic field. The dipole component of an object's magnetic field is symmetric about the direction of its magnetic dipole moment, and decreases as the inverse cube of the distance from the object.
Contents
Two definitions of moment
In text books, two complementary approaches have been used to define magnetic moments. In pre-1930's textbooks, they were defined using magnetic poles.[1] Most recent textbooks define it in terms of Ampèrian currents.
Magnetic pole definition
The sources of magnetic moments in materials can be represented by poles in analogy to electrostatics. Consider a bar magnet which has magnetic poles of equal magnitude but opposite polarity. Each pole is the source of magnetic force which weakens with distance. Since magnetic poles always come in pairs, their forces partially cancel each other because while one pole pulls, the other repels. This cancellation is greatest when the poles are close to each other i.e. when the bar magnet is short. The magnetic force produced by a bar magnet, at a given point in space, therefore depends on two factors: on both the strength p of its poles, and on the vector
 separating them. The moment is defined as[1]
separating them. The moment is defined as[1]It points in the direction from South to North pole. The analogy with electric dipoles should not be taken too far because magnetic dipoles are associated with angular momentum (see Magnetic moment and angular momentum). Nevertheless, magnetic poles are very useful for magnetostatic calculations, particularly in applications to ferromagnets.[1] Practitioners using the magnetic pole approach generally represent the magnetic field by the irrotational field
 , in analogy to the electric field
, in analogy to the electric field  .
.Current loop definition
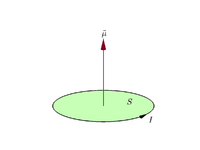
Suppose a planar closed loop carries an electric current I and has vector area
 (x, y, and z coordinates of this vector are the areas of projections of the loop onto the yz, zx, and xy planes). Its magnetic moment
(x, y, and z coordinates of this vector are the areas of projections of the loop onto the yz, zx, and xy planes). Its magnetic moment  , vector, is defined as:
, vector, is defined as:By convention, the direction of the vector area is given by the right hand grip rule (curling the fingers of one's right hand in the direction of the current around the loop, when the palm of the hand is "touching" the loop's outer edge, and the straight thumb indicates the direction of the vector area and thus of the magnetic moment). [2]
If the loop is not planar, the moment is given as
In the most general case of an arbitrary current distribution in space, the magnetic moment of such a distribution can be found from the following equation:
where
 is the position vector pointing from the origin to the location of the volume element, and
is the position vector pointing from the origin to the location of the volume element, and  is the current density vector at that location.
is the current density vector at that location.The above equation can be used for calculating a magnetic moment of any assembly of moving charges, such as a spinning charged solid, by substituting
where ρ is the electric charge density at a given point and
 is the instantaneous linear velocity of that point.
is the instantaneous linear velocity of that point.For example, the magnetic moment produced by an electric charge moving along a circular path is
 ,
,
where
 is the position of the charge q relative to the center of the circle and
is the position of the charge q relative to the center of the circle and  is the instantaneous velocity of the charge.
is the instantaneous velocity of the charge.Practitioners using the current loop model generally represent the magnetic field by the solenoidal field
 , analogous to the electrostatic field
, analogous to the electrostatic field  .
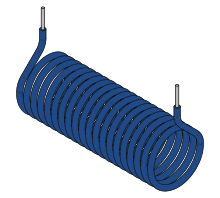
.Magnetic moment of a solenoid
A generalization of the above current loop is a multi-turn coil, or solenoid. Its moment is the vector sum of the moments of individual turns. If the solenoid has N identical turns (single-layer winding),
Units
The unit for magnetic moment is not a base unit in the International System of Units (SI) and it can be represented in more than one way. For example, in the current loop definition, the area is measured in square meters and I is measured in amperes, so the magnetic moment is measured in ampere–square meters (A m2). In the equation for torque on a moment, the torque is measured in joules and the magnetic field in tesla, so the moment is measured in Joules per Tesla (J T − 1). These two representations are equivalent:
In the CGS system, there are several different sets of electromagnetism units, of which the main ones are ESU, Gaussian, and EMU. Among these, there are two alternative (non-equivalent) units of magnetic dipole moment in CGS:
and (more frequently used)
The ratio of these two non-equivalent CGS units (EMU/ESU) is equal exactly to the speed of light in free space, expressed in cm/s.
All formulas in this article are correct in SI units, but in other unit systems, the formulas may need to be changed. For example, in SI units, a loop of current with current I and area A has magnetic moment I×A (see below), but in Gaussian units the magnetic moment is I×A/c.
Effects of an external magnetic field on a magnetic moment
Force on a moment
See also: force between magnetsA magnetic moment in an externally-produced magnetic field has a potential energy U:
In a case when the external magnetic field is non-uniform, there will be a force, proportional to the magnetic field gradient, acting on the magnetic moment itself. There has been some discussion on how to calculate the force acting on a magnetic dipole. There are two expressions for the force acting on a magnetic dipole, depending on whether the model used for the dipole is a current loop or two monopoles (analogous to the electric dipole).[3] The force obtained in the case of a current loop model is
In the case of a pair of monopoles are used (i.e. electric dipole model)
and one can be put in terms of the other via the relation
In all these expressions
 is the dipole and
is the dipole and  is the magnetic field at its position. Note that if there are no currents or time-varying electrical fields
is the magnetic field at its position. Note that if there are no currents or time-varying electrical fields  and the two expressions agree.
and the two expressions agree.An electron, nucleus, or atom placed in a uniform magnetic field will precess with a frequency known as the Larmor frequency. See Resonance.
Torque on a moment
The magnetic moment can also be defined as a vector relating the aligning torque on the object from an externally applied magnetic field to the field vector itself. The relationship is given by [4]
where
 is the torque and
is the torque and  is the magnetic field.
is the magnetic field.Magnetic moment and angular momentum
The magnetic moment has a close connection with angular momentum called the gyromagnetic effect. This effect is expressed on a macroscopic scale in the Einstein-de Haas effect, or "rotation by magnetization," and its inverse, the Barnett effect, or "magnetization by rotation."[4] In particular, when a magnetic moment is subject to a torque in a magnetic field that tends to align it with the applied magnetic field, the moment precesses (rotates about the axis of the applied field). This is a consequence of the angular momentum associated with the moment.
Viewing a magnetic dipole as a rotating charged sphere brings out the close connection between magnetic moment and angular momentum. Both the magnetic moment and the angular momentum increase with the rate of rotation of the sphere. The ratio of the two is called the gyromagnetic ratio, usually denoted by the symbol γ.[5] [6]
For a spinning charged solid with a uniform charge density to mass density ratio, the gyromagnetic ratio is equal to half the charge-to-mass ratio. This implies that a more massive assembly of charges spinning with the same angular momentum will have a proportionately weaker magnetic moment, compared to its lighter counterpart. Even though atomic particles cannot be accurately described as spinning charge distributions of uniform charge-to-mass ratio, this general trend can be observed in the atomic world, where the intrinsic angular momentum (spin) of each type of particle is a constant: a small half-integer times the reduced Planck constant
 . This is the basis for defining the magnetic moment units of Bohr magneton (assuming charge-to-mass ratio of the electron) and nuclear magneton (assuming charge-to-mass ratio of the proton).
. This is the basis for defining the magnetic moment units of Bohr magneton (assuming charge-to-mass ratio of the electron) and nuclear magneton (assuming charge-to-mass ratio of the proton).Magnetic dipoles
A magnetic dipole is the limit of either a current loop or a pair of poles as the dimensions of the source are reduced to zero while keeping the moment constant. As long as these limits only applies to fields far from the sources, they are equivalent. However, the two models give different predictions for the internal field (see below).
External magnetic field produced by a magnetic dipole moment
Any system possessing a net magnetic dipole moment
 will produce a dipolar magnetic field (described below) in the space surrounding the system. While the net magnetic field produced by the system can also have higher-order multipole components, those will drop off with distance more rapidly, so that only the dipolar component will dominate the magnetic field of the system at distances far away from it.
will produce a dipolar magnetic field (described below) in the space surrounding the system. While the net magnetic field produced by the system can also have higher-order multipole components, those will drop off with distance more rapidly, so that only the dipolar component will dominate the magnetic field of the system at distances far away from it.The vector potential of magnetic field produced by magnetic moment m is
and magnetic flux density is
Alternatively one can obtain the scalar potential first from the magnetic pole perspective,
and hence magnetic field strength is
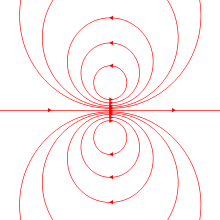
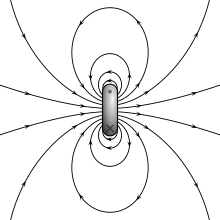
The magnetic field of an ideal magnetic dipole is depicted on the left.
Internal magnetic field of a dipole
The two models for a dipole (current loop and magnetic poles) give the same predictions for the magnetic field far from the source. However, inside the source region they give different predictions. The magnetic field between poles (see figure for Magnetic pole definition) is in the opposite direction to the magnetic moment (which points from the negative charge to the positive charge), while inside a current loop it is in the same direction (see the figure to the right). Clearly, the limits of these fields must also be different as the sources shrink to zero size. This distinction only matters if the dipole limit is used to calculate fields inside a magnetic material.
If a magnetic dipole is formed by making a current loop smaller and smaller, but keeping the product of current and area constant, the limiting field is
Unlike the expressions in the previous section, this limit is correct for the internal field of the dipole.
If a magnetic dipole is formed by taking a "north pole" and a "south pole", bringing them closer and closer together but keeping the product of magnetic pole-charge and distance constant, the limiting field is
These fields are related by
 , where
, whereis the magnetization.
Forces between two magnetic dipoles
If
 in the previous equations is replaced with the expression of the field of a magnetic dipole under the approximation for distances bigger than the characteristic length of the dipole.[7] Namely,
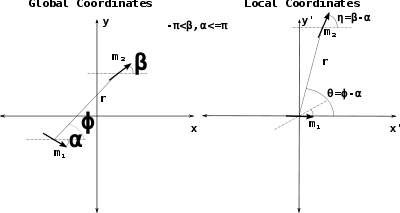
in the previous equations is replaced with the expression of the field of a magnetic dipole under the approximation for distances bigger than the characteristic length of the dipole.[7] Namely,where the variables r and θ are measured in a frame of reference with origin in
 and oriented in such a way that
and oriented in such a way that  lies in the x-axis. This frame is called Local coordinates and is shown in the Figure on the right.
lies in the x-axis. This frame is called Local coordinates and is shown in the Figure on the right.The final formulas are shown next. They are expressed in the global coordinate system,
Using vector notation, the above equations can be written as,
where
 is the distance-vector from dipole moment
is the distance-vector from dipole moment  to dipole moment
to dipole moment  , with
, with  , and where
, and where  is the force acting on
is the force acting on  . The force acting on
. The force acting on  is in opposite direction.
is in opposite direction.The torque is straightforward to obtain from the formula
which gives
Examples of magnetic moments
Two kinds of magnetic sources
Fundamentally, contributions to any system's magnetic moment may come from sources of two kinds: (1) motion of electric charges, such as electric currents, and (2) the intrinsic magnetism of elementary particles, such as the electron.
Contributions due to the sources of the first kind can be calculated from knowing the distribution of all the electric currents (or, alternatively, of all the electric charges and their velocities) inside the system, by using the formulas below. On the other hand, the magnitude of each elementary particle's intrinsic magnetic moment is a fixed number, often measured experimentally to a great precision. For example, any electron's magnetic moment is measured to be −9.284764×10−24 J/T.[9] The direction of the magnetic moment of any elementary particle is entirely determined by the direction of its spin (the minus in front of the value above indicates that any electron's magnetic moment is antiparallel to its spin).
The net magnetic moment of any system is a vector sum of contributions from one or both types of sources. For example, the magnetic moment of an atom of hydrogen-1 (the lightest hydrogen isotope, consisting of a proton and an electron) is a vector sum of the following contributions: (1) the intrinsic moment of the electron, (2) the orbital motion of the electron around the proton, (3) the intrinsic moment of the proton. Similarly, the magnetic moment of a bar magnet is the sum of the intrinsic and orbital magnetic moments of the unpaired electrons of the magnet's material.
Magnetic moment of an atom
For an atom, individual electron spins are added to get a total spin, and individual orbital angular momenta are added to get a total orbital angular momentum. These two then are added using angular momentum coupling to get a total angular momentum. The magnitude of the atomic dipole moment is then:[10]
where J is the total angular momentum quantum number, gJ is the Landé g-factor, and μB is the Bohr magneton. The component of this magnetic moment along the direction of the magnetic field is then:[11]
- mAtom(z) = − mgJμB
where m is called the magnetic quantum number or the equatorial quantum number, which can take on any of 2J + 1 values: − J, − (J − 1),
 , (J − 1), J.[12] The negative sign occurs because electrons have negative charge.
, (J − 1), J.[12] The negative sign occurs because electrons have negative charge.Because of the angular momentum, the dynamics of a magnetic dipole in a magnetic field differs from that of an electric dipole in an electric field. The field does exert a torque on the magnetic dipole tending to align it with the field. However, torque is proportional to rate of change of angular momentum, so precession occurs: the direction of spin changes. This behavior is described by the Landau-Lifshitz-Gilbert equation:[13][14]
where γ is gyromagnetic ratio,
 is magnetic moment, λ is damping coefficient and
is magnetic moment, λ is damping coefficient and  is effective magnetic field (the external field plus any self-field), and
is effective magnetic field (the external field plus any self-field), and  is the vector cross product. The first term describes precession of the moment about the effective field, while the second is a damping term related to dissipation of energy caused by interaction with surroundings.
is the vector cross product. The first term describes precession of the moment about the effective field, while the second is a damping term related to dissipation of energy caused by interaction with surroundings.Magnetic moment of an electron
Electrons and many elementary particles also have intrinsic magnetic moments, an explanation of which requires a quantum mechanical treatment and relates to the intrinsic angular momentum of the particles as discussed in the article electron magnetic dipole moment. It is these intrinsic magnetic moments that give rise to the macroscopic effects of magnetism, and other phenomena, such as electron paramagnetic resonance.
The magnetic moment of the electron is
where
- μB is the Bohr magneton,
 is electron spin,
is electron spin,
and the electron g-factor is
- gS = 2 in Dirac mechanics, but is slightly larger,
 in reality due to quantum electrodynamics effects.
in reality due to quantum electrodynamics effects.
Again it is important to notice that
 is a negative constant multiplied by the spin, so the magnetic moment of the electron is antiparallel to the spin. This can be understood with the following classical picture: if we imagine that the spin angular momentum is created by the electron mass spinning around some axis, the electric current that this rotation creates circulates in the opposite direction, because of the negative charge of the electron; such current loops produce a magnetic moment which is antiparallel to the spin. Hence, for a positron (the anti-particle of the electron) the magnetic moment is parallel to its spin.
is a negative constant multiplied by the spin, so the magnetic moment of the electron is antiparallel to the spin. This can be understood with the following classical picture: if we imagine that the spin angular momentum is created by the electron mass spinning around some axis, the electric current that this rotation creates circulates in the opposite direction, because of the negative charge of the electron; such current loops produce a magnetic moment which is antiparallel to the spin. Hence, for a positron (the anti-particle of the electron) the magnetic moment is parallel to its spin.Magnetic moment of a nucleus
The nuclear system is a complex physical system consisting of nucleons, i.e., protons and neutrons. The quantum mechanical properties of the nucleons include the spin among others. Since the electromagnetic moments of the nucleus depend on the spin of the individual nucleons, one can look at these properties with measurements of nuclear moments, and more specifically the nuclear magnetic dipole moment.
Most common nuclei exist in their ground state, although nuclei of some isotopes have long-lived excited states. Each energy state of a nucleus of a given isotope is characterized by a well-defined magnetic dipole moment, the magnitude of which is a fixed number, often measured experimentally to a great precision. This number is very sensitive to the individual contributions from nucleons, and a measurement or prediction of its value can reveal important information about the content of the nuclear wave function. There are several theoretical models that predict the value of the magnetic dipole moment and a number of experimental techniques aiming to carry out measurements in nuclei along the nuclear chart.
Magnetic moment of a molecule
Any molecule has a well-defined magnitude of magnetic moment, which may depend on the molecule's energy state. Typically, the overall magnetic moment of a molecule is a combination of the following contributions, in the order of their typical strength:
- magnetic moments due to its unpaired electron spins (paramagnetic contribution), if any
- orbital motion of its electrons, which in the ground state is often proportional to the external magnetic field (diamagnetic contribution)
- the combined magnetic moment of its nuclear spins, which depends on the nuclear spin configuration.
Examples of molecular magnetism
- Oxygen molecule, O2, exhibits strong paramagnetism, due to unpaired spins of its outermost two electrons.
- Carbon dioxide molecule, CO2, mostly exhibits diamagnetism, a much weaker magnetic moment of the electron orbitals that is proportional to the external magnetic field. In the rare instance when a magnetic isotope, such as 13C or 17O, is present, it will contribute its nuclear magnetism to the molecule's magnetic moment.
- Hydrogen molecule, H2, in a weak (or zero) magnetic field exhibits nuclear magnetism, and can be in a para- or an ortho- nuclear spin configuration.
Elementary particles
In atomic and nuclear physics, the symbol μ represents the magnitude of the magnetic moment, often measured in Bohr magnetons or nuclear magnetons, associated with the intrinsic spin of the particle and/or with the orbital motion of the particle in a system. Values of the intrinsic magnetic moments of some particles are given in the table below:
Intrinsic magnetic moments and spins of some elementary particles [15] Particle Magnetic dipole moment in SI units (10−27 J/T) Spin quantum number (dimensionless) electron -9284.764 1/2 proton 14.106067 1/2 neutron -9.66236 1/2 muon -44.904478 1/2 deuteron 4.3307346 1 triton 15.046094 1/2 For relation between the notions of magnetic moment and magnetization see magnetization.
See also
- Magnetism
- Magnetic dipole models
- Dipole
- Electric dipole moment
- Magnetization
- Magnetic field
- Magnetic susceptibility
- Magnetic dipole-dipole interaction
- Landau-Lifshitz-Gilbert equation
References and notes
- ^ a b c Brown, Jr., William Fuller. Magnetostatic Principles in Ferromagnetism. North-Holland.
- ^ Feynman, Richard P.; Leighton, Robert B.; Sands, Matthew (2006). The Feynman Lectures on Physics. 2. ISBN 0-8053-9045-6.
- ^ Boyer, Timothy H. (1988). "The Force on a Magnetic Dipole". American Journal of Physics 56 (8): 688–692. Bibcode 1988AmJPh..56..688B. doi:10.1119/1.15501.
- ^ a b B. D. Cullity, C. D. Graham (2008). Introduction to Magnetic Materials (2 ed.). Wiley-IEEE Press. p. 103. ISBN 0471477419. http://books.google.com/?id=ixAe4qIGEmwC&pg=PA103.
- ^ Uwe Krey, Anthony Owen (2007). Basic Theoretical Physics. Springer. pp. 151–152. ISBN 3540368043. http://books.google.com/?id=xZ_QelBmkxYC&pg=PA151.
- ^ Richard B. Buxton (2002). Introduction to functional magnetic resonance imaging. Cambridge University Press. p. 136. ISBN 0521581133. http://books.google.com/?id=6XVu0NKzgekC&pg=PA136.
- ^ Schill, R. A. (2003). "General relation for the vector magnetic field of a circular current loop: A closer look". IEEE Transactions on Magnetics 39 (2): 961–967. Bibcode 2003ITM....39..961S. doi:10.1109/TMAG.2003.808597.
- ^ Furlani, Edward P. (2001). Permanent Magnet and Electromechanical Devices: Materials, Analysis, and Applications. Academic Press. p. 140. ISBN 0122699513. http://books.google.com/?id=irsdLnC5SrsC&dq=permanent+magnet+and+electromechanical+devices&printsec=frontcover&q=3.130.
- ^ "CODATA value: electron magnetic moment". http://physics.nist.gov/cgi-bin/cuu/Value?muem.
- ^ RJD Tilley (2004). Understanding Solids. John Wiley and Sons. p. 368. ISBN 0470852755. http://books.google.com/?id=ZVgOLCXNoMoC&pg=PA368.
- ^ Paul Allen Tipler, Ralph A. Llewellyn (2002). Modern Physics (4 ed.). Macmillan. p. 310. ISBN 0716743450. http://books.google.com/?id=tpU18JqcSNkC&pg=PA310.
- ^ JA Crowther (2007). Ions, Electrons and Ionizing Radiations (reprinted Cambridge (1934) 6 ed.). Rene Press. p. 277. ISBN 1406720399. http://books.google.com/?id=H_sft9-zm5AC&pg=PA277.
- ^ Stuart Alan Rice (2004). Advances in chemical physics. Wiley. pp. 208 ff. ISBN 0471445282. http://books.google.com/?id=wK3Vhq-VnBQC&pg=PA208.
- ^ Marcus Steiner (2004). Micromagnetism and Electrical Resistance of Ferromagnetic Electrodes for Spin Injection Devices. Cuvillier Verlag. p. 6. ISBN 3865371760. http://books.google.com/?id=tnX1edkCB-wC&pg=PA6.
- ^ See NIST's Fundamental Physical Constants website http://physics.nist.gov/cgi-bin/cuu/Results?search_for=+magnetic+moment
Categories:- Magnetostatics
- Magnetism
- Electric and magnetic fields in matter
- Physical quantities
Wikimedia Foundation. 2010.


















![\mathbf{B}(\mathbf{x})=\frac{\mu_0}{4\pi}\left[\frac{3\mathbf{n}(\mathbf{n}\cdot \mathbf{m})-\mathbf{m}}{|\mathbf{x}|^3} + \frac{8\pi}{3}\mathbf{m}\delta(\mathbf{x})\right].](a/50a8cec424e6fc9fd4bc0abdfb3d59a2.png)
![\mathbf{H}(\mathbf{x}) =\frac{1}{4\pi}\left[\frac{3\mathbf{n}(\mathbf{n}\cdot \mathbf{m})-\mathbf{m}}{|\mathbf{x}|^3} - \frac{4\pi}{3}\mathbf{m}\delta(\mathbf{x})\right].](1/ac1ba459985f81ddc05c1109717692b9.png)



![F_r(\mathbf{r}, \alpha, \beta) = - \frac{3 \mu_0}{4 \pi}\frac{m_2 m_1}{r^4}\left[2\cos(\phi - \alpha)\cos(\phi - \beta)- \sin(\phi - \alpha)\sin(\phi - \beta)\right]](9/6b952b63d35a82e4f2855a0e98decfe8.png)

![\mathbf{F}(\mathbf{r}, \mathbf{m}_1, \mathbf{m}_2) = \dfrac{3 \mu_0}{4 \pi r^5}\left[(\mathbf{m}_1\cdot\mathbf{r})\mathbf{m}_2 + (\mathbf{m}_2\cdot\mathbf{r})\mathbf{m}_1 + (\mathbf{m}_1\cdot\mathbf{m}_2)\mathbf{r} - \dfrac{5(\mathbf{m}_1\cdot\mathbf{r})(\mathbf{m}_2\cdot\mathbf{r})}{r^2}\mathbf{r}\right]](b/66b404b97b7f1153fc73f162681dffe5.png)

![\boldsymbol{\tau}= \frac{\mu_0}{4 \pi}\frac{m_1 m_2}{r^3}\left[3\cos(\phi-\alpha)\sin(\phi-\beta)+\sin(\beta-\alpha)\right]](e/40e320ea3a1d6aa16171d66ff680fcd1.png)


