- Charles-Augustin de Coulomb
-
Charles-Augustin de Coulomb 
Portrait by Hippolyte LecomteBorn 14 June 1736
Angoulême, FranceDied 23 August 1806 (aged 70)
Paris, FranceNationality French Fields Physics Known for Coulomb's law Charles-Augustin de Coulomb (14 June 1736 – 23 August 1806) was a French physicist. He is best known for developing Coulomb's law, the definition of the electrostatic force of attraction and repulsion. The SI unit of charge, the coulomb, was named after him.
Contents
Life
Coulomb was born in Angoulême, France, to an affluent family. His father, Henri Coulomb, was inspector of the Royal Fields in Montpellier. His mother, Catherine Bajet, came from a wealthy family in the wool trade. When Coulomb was a boy, the family moved to Paris and there Coulomb studied at the prestigious Collège des Quatre-Nations. The courses he studied in mathematics there, under Pierre Charles Monnier, left him determined to pursue mathematics and similar subjects as a career. From 1757 to 1759 he joined his father's family in Montpellier and took part in the work of the academy of the city, directed by the mathematician Augustin Danyzy. With his father's approval, Coulomb returned to Paris in 1759 where he was successful in the entrance examination for the military school at Mézières.
After he left the school in 1761, Coulomb initially took part in the survey for the British coastal charts and was then sent on a mission to Martinique in 1764 to take part in the construction of the Fort Bourbon under the orders of the lieutenant-colonel of Rochemore, as the French colony was insulated in the middle of the English and Spanish possessions following the Seven Years' War. Coulomb spent eight years directing the work, contracting tropical fever. He carried out several experiments on the resistance of masonries and the behaviour of the walls of escarpe (supportings), which were inspired by the ideas of Pieter van Musschenbroek on friction.
Upon his return to France, with the rank of Captain, he was employed at La Rochelle, the Isle of Aix and Cherbourg. He discovered an inverse relationship of the force between electric charges and the square of its distance, later named after him as Coulomb's law.
In 1781, he was stationed at Paris. On the outbreak of the Revolution in 1789, he resigned his appointment as intendant des eaux et fontaines and retired to a small estate which he possessed at Blois. He was recalled to Paris for a time in order to take part in the new determination of weights and measures, which had been decreed by the Revolutionary government. He became one of the first members of the French National Institute and was appointed inspector of public instruction in 1802. His health was already very feeble and four years later he died in Paris.
Coulomb leaves a legacy as a pioneer in the field of geotechnical engineering for his contribution to retaining wall design. His name is one of the 72 names inscribed on the Eiffel Tower.
Research
In 1784, his memoir Recherches théoriques et expérimentales sur la force de torsion et sur l'élasticité des fils de metal[1] (Theoretical research and experimentation on torsion and the elasticity of metal wire) appeared. This memoir contained the results of Coulomb's experiments on the torsional force for metal wires. His general result is,
- "... the moment of the torque is, for wires of the same metal, proportional to the torsional angle, the fourth power of the diameter and the inverse of the length of the wire..."
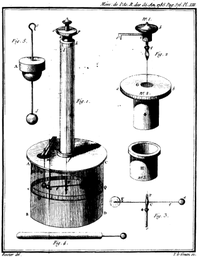
It also contained a detailed description of different forms of his torsion balance. He used the instrument with great success for the experimental investigation of the distribution of charge on surfaces, of the laws of electrical and magnetic force and of the mathematical theory of which he may also be regarded as the founder.
In 1785, Coulomb presented his three reports on Electricity and Magnetism:
- Premier Mémoire sur l’Électricité et le Magnétisme.[2] In this publication, Coulomb describes "How to construct and use an electric balance (torsion balance) based on the property of the metal wires of having a reaction torsion force proportional to the torsion angle." Coulomb also experimentally determined the law that explains how "two bodies electrified of the same kind of Electricity exert on each other." On page 574 he states:
Il résulte donc de ces trois essais, que l'action répulsive que les deux balles électrifées de la même nature d'électricité exercent l'une sur l'autre, suit la raison inverse du carré des distances.
Translation: It follows therefore from these three tests, that the repulsive force that the two balls -- [which were] electrified with the same kind of electricity -- exert on each other, follows the inverse proportion of the square of the distance.
- Second Mémoire sur l’Électricité et le Magnétisme.[3] In this publication, Coulomb carries out the "determination according to which laws both the Magnetic and the Electric fluids act, either by repulsion or by attraction." On page 579, he states that the attractive force between two oppositely charged spheres is proportional to the product of the quantities of charge on the spheres and is inversely proportional to the square of the distance between the spheres.
- Troisième Mémoire sur l’Électricité et le Magnétisme.[4] "On the quantity of Electricity that an isolated body loses in a certain time period, either by contact with less humid air or in the supports more or less idio-electric."
Four subsequent reports were published in the following years:
- Quatrième Mémoire[5] "Where two principal properties of the electric fluid are demonstrated: first, that this fluid does not expand into any object according to a chemical affinity or by an elective attraction, but that it divides itself between different objects brought into contact; second, that in conducting objects, the fluid, having achieved a state of stability, expands on the surface of the body and does not penetrate into the interior." (1786)
- Cinquième Mémoire[6] "On the manner in which the electric fluid divides itself between conducting objects brought into contact and the distribution of this fluid on the different parts of the surface of this object." (1787)
- Sixième Mémoire[7] "Continuation of research into the distribution of the electric fluid between several conductors. Determination of electric density at different points on the surface of these bodies." (1788)
- Septième Mémoire[8] "On magnetism" (1789)
Coulomb explained the laws of attraction and repulsion between electric charges and magnetic poles, although he did not find any relationship between the two phenomena. He thought that the attraction and repulsion were due to different kinds of fluids.
See also
- Coulomb friction
- Coulomb's Law
- Coulomb's inverse square law
References
- ^ Coulomb (1784) "Recherches théoriques et expérimentales sur la force de torsion et sur l'élasticité des fils de metal," Histoire de l’Académie Royale des Sciences, pages 229-269.
- ^ Coulomb (1785a) "Premier mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pages 569-577.
- ^ Coulomb (1785b) "Second mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pages 578-611.
- ^ Coulomb (1785c) "Troisième mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pages 612-638.
- ^ Coulomb (1786) "Quatrième mémoire sur l’électricité," Histoire de l’Académie Royale des Sciences, pages 67-77.
- ^ Coulomb (1787) "Cinquième mémoire sur l’électricité," Histoire de l’Académie Royale des Sciences, pages 421-467.
- ^ Coulomb (1788) "Sixième mémoire sur l’électricité," Histoire de l’Académie Royale des Sciences, pages 617-705.
- ^ Coulomb (1789) "Septième mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pages 455-505.
External links
- Théorie des machines simples (1821)
- Collection de mémoires relatifs à la physique (1884)
- French National Library The Mémoires of Coulomb available in pdf format.
- O'Connor, John J.; Robertson, Edmund F., "Charles-Augustin de Coulomb", MacTutor History of Mathematics archive, University of St Andrews, http://www-history.mcs.st-andrews.ac.uk/Biographies/Coulomb.html.
See also
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed (1911). Encyclopædia Britannica (11th ed.). Cambridge University Press.Scientists whose names are used as SI units Base units Derived units Blaise Pascal • Isaac Newton • Anders Celsius • Charles-Augustin de Coulomb • James Watt • Alessandro Volta • Georg Simon Ohm • Michael Faraday • Joseph Henry • Wilhelm Eduard Weber • Ernst Werner von Siemens • James Prescott Joule • Antoine Henri Becquerel • Nikola Tesla • Heinrich Rudolf Hertz • Rolf Maximilian Sievert • Louis Harold GrayNon SI units Scientists whose names are used as non SI unitsConstants List of scientists whose names are used in physical constantsScientists whose names are used in physical constants physical constants Isaac Newton · Charles-Augustin de Coulomb · Amedeo Avogadro · Michael Faraday · Johann Josef Loschmidt · Johann Jakob Balmer · Joseph Stefan · Ludwig Boltzmann · Johannes Rydberg · Joseph John Thomson · Max Planck · Wilhelm Wien · Otto Sackur · Niels Bohr · Edwin Hubble · Hugo Tetrode · Douglas Hartree · Enrico Fermi · Brian David Josephson · Klaus von KlitzingList of scientists whose names are used as SI units and non SI unitsCategories:- 1736 births
- 1806 deaths
- People from Angoulême
- University of Paris alumni
- French physicists
- Geotechnical engineers
- French civil engineers
- French Roman Catholics
- Members of the French Academy of Sciences
Wikimedia Foundation. 2010.