- Coulomb's law
-
Electromagnetism 
Electricity · Magnetism Lorentz force law · emf · Electromagnetic induction · Faraday’s law · Lenz's law · Displacement current · Maxwell's equations · EM field · Electromagnetic radiation · Liénard–Wiechert potential · Maxwell tensor · Eddy currentCoulomb's law or Coulomb's inverse-square law, is a law of physics describing the electrostatic interaction between electrically charged particles. It was first published in 1785 by French physicist Charles Augustin de Coulomb and was essential to the development of the theory of electromagnetism.[1][2] Nevertheless, in 1767 Joseph Priestley of England conjectured that the force between charges varied as the inverse squared of the distance.[3][4] In 1769, Scottish physicist John Robison announced that according to his measurements, the force of repulsion between two spheres with charges of the same sign varied as x-2.06.[5] The dependence of the force between charged bodies upon both distance and charge had been discovered, but not published, in the early 1770s by Henry Cavendish of England, prior to Coulomb's works.[6]
Contents
Basic equation
The scalar form of Coulomb's law is an expression for the magnitude and sign of the electrostatic force between two idealized point charges, small in size compared to their separation. This force (F) acting simultaneously on point charges (q1) and (q2), is given by
where r is the separation distance and ke is a proportionality constant. A positive force implies it is repulsive, while a negative force implies it is attractive.[7] The proportionality constant ke, called the Coulomb constant (sometimes called the Coulomb force constant), is related to defined properties of space and can be calculated based on the speed of light to be exactly:[8]
Coulomb's law states that: "The magnitude of the Electrostatics force of interaction between two point charges is directly proportional to the scalar multiplication of the magnitudes of charges and inversely proportional to the square of the distances between them."
In SI units, the meter is defined such that the speed of light in vacuum (or electromagnetic waves, in general), denoted c,[9] is exactly 299,792,458 m·s−1[10], and the magnetic constant (μ0) is set at 4π × 10−7 H·m−1.[11] In agreement with electromagnetic theory, requiring that
the value for the electric constant (ε0) is derived to be ε0 = 1/(μ0c2) ≈ 8.85418782×10−12 F·m−1.[12] In electrostatic units and Gaussian units, the unit charge (esu or statcoulomb) is defined in such a way that the Coulomb constant is 1 and dimensionless.
In the more useful vector-form statement, the force in the equation is a vector force acting on either point charge, so directed as to push it away from the other point charge; the right-hand side of the equation, in this case, must have an additional product term of a unit vector pointing in one of two opposite directions, e.g., from q1 to q2 if the force is acting on q2; the charges may have either sign and the sign of their product determines the ultimate direction of that force. Thus, the vector force pushing the charges away from each other (pulling towards each other if negative) is directly proportional to the product of the charges and inversely proportional to the square of the distance between them. The square of the distance part arises from the fact that the force field due to an isolated point charge is uniform in all directions and gets "diluted" with distance as much as the area of a sphere centered on the point charge expands with its radius.
The law of superposition allows this law to be extended to include any number of point charges, to derive the force on any one point charge by a vector addition of these individual forces acting alone on that point charge. The resulting vector happens to be parallel to the electric field vector at that point, with that point charge (or "test charge") removed.
Coulomb's law can also be interpreted in terms of atomic units with the force expressed in Hartrees per Bohr radius, the charge in terms of the elementary charge, and the distances in terms of the Bohr radius.
Electric field
Main article: Electric fieldIt follows from the Coulomb's Law that the magnitude of the electric field (E) created by a single point charge (q) at a certain distance (r) is given by:
For a positive charge, the direction of the electric field points along lines directed radially away from the location of the point charge, while the direction is the opposite for a negative charge. The SI units of electric field are volts per meter or newtons per coulomb.
Vector form
In order to obtain both the magnitude and direction of the force on a charge, q1 at position
 , experiencing a field due to the presence of another charge, q2 at position
, experiencing a field due to the presence of another charge, q2 at position  , the full vector form of Coulomb's law is required.
, the full vector form of Coulomb's law is required.where r is the separation of the two charges. This is simply the scalar definition of Coulomb's law with the direction given by the unit vector,
 , parallel with the line from charge q2 to charge q1.[13]
, parallel with the line from charge q2 to charge q1.[13]If both charges have the same sign (like charges) then the product q1q2 is positive and the direction of the force on q1 is given by
 ; the charges repel each other. If the charges have opposite signs then the product q1q2 is negative and the direction of the force on q1 is given by
; the charges repel each other. If the charges have opposite signs then the product q1q2 is negative and the direction of the force on q1 is given by  ; the charges attract each other.
; the charges attract each other.System of discrete charges
The principle of linear superposition may be used to calculate the force on a small test charge, q, due to a system of N discrete charges:
where qi and
 are the magnitude and position respectively of the ith charge,
are the magnitude and position respectively of the ith charge,  is a unit vector in the direction of
is a unit vector in the direction of  (a vector pointing from charge qi to charge q), and Ri is the magnitude of
(a vector pointing from charge qi to charge q), and Ri is the magnitude of  (the separation between charges qi and q).[13]
(the separation between charges qi and q).[13]Continuous charge distribution
For a charge distribution an integral over the region containing the charge is equivalent to an infinite summation, treating each infinitesimal element of space as a point charge dq.
For a linear charge distribution (a good approximation for charge in a wire) where
 gives the charge per unit length at position
gives the charge per unit length at position  , and
, and  is an infinitesimal element of length,
is an infinitesimal element of length, .[14]
.[14]
For a surface charge distribution (a good approximation for charge on a plate in a parallel plate capacitor) where
 gives the charge per unit area at position
gives the charge per unit area at position  , and
, and  is an infinitesimal element of area,
is an infinitesimal element of area,For a volume charge distribution (such as charge within a bulk metal) where
 gives the charge per unit volume at position
gives the charge per unit volume at position  , and
, and  is an infinitesimal element of volume,
is an infinitesimal element of volume,The force on a small test charge
 at position
at position  is given by
is given byGraphical representation
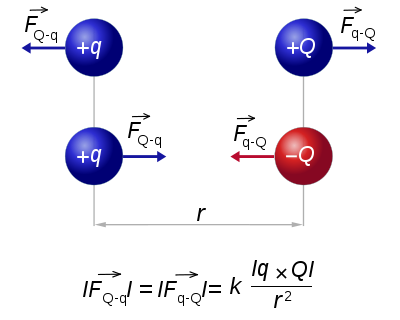
Below is a graphical representation of Coulomb's law, when q1q2 > 0. The vector
 is the force experienced by q1. The vector
is the force experienced by q1. The vector  is the force experienced by q2. Their magnitudes will always be equal. The vector
is the force experienced by q2. Their magnitudes will always be equal. The vector  is the displacement vector between two charges (q1 and q2).
is the displacement vector between two charges (q1 and q2). A graphical representation of Coulomb's law.
A graphical representation of Coulomb's law.Electrostatic approximation
In either formulation, Coulomb’s law is fully accurate only when the objects are stationary, and remains approximately correct only for slow movement. These conditions are collectively known as the electrostatic approximation. When movement takes place, magnetic fields that alter the force on the two objects are produced. The magnetic interaction between moving charges may be thought of as a manifestation of the force from the electrostatic field but with Einstein’s theory of relativity taken into consideration.
Atomic forces
Coulomb's law holds even within the atoms, correctly describing the force between the positively charged nucleus and each of the negatively charged electrons. This simple law also correctly accounts for the forces that bind atoms together to form molecules and for the forces that bind atoms and molecules together to form solids and liquids.
Table of derived quantities
Particle property Relationship Field property Vector quantity Force (on 1 by 2) 

Electric field (at 1 by 2) 
Relationship 

Scalar quantity Potential energy (at 1 by 2) 

Potential (at 1 by 2) 
See also
- Biot–Savart law
- Method of image charges
- Electric field
- Electric constant
- Coulomb, the SI unit of electric charge named after Charles Augustin de Coulomb
- Electromagnetic force
- Molecular modelling
- Static forces and virtual-particle exchange
- Darwin Lagrangian
- Newton's Law of Universal Gravitation, which uses a similar structure, but for mass instead of charge.
Notes
- ^ In -- Coulomb (1785a) "Premier mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pages 569-577 -- Coulomb studied the repulsive force between bodies having electrical charges of the same sign:
Page 574 : Il résulte donc de ces trois essais, que l'action répulsive que les deux balles électrifées de la même nature d'électricité exercent l'une sur l'autre, suit la raison inverse du carré des distances.
In -- Coulomb (1785b) "Second mémoire sur l’électricité et le magnétisme," Histoire de l’Académie Royale des Sciences, pages 578-611. -- Coulomb showed that oppositely charged bodies obey an inverse-square law of attraction.Translation : It follows therefore from these three tests, that the repulsive force that the two balls -- [that were] electrified with the same kind of electricity -- exert on each other, follows the inverse proportion of the square of the distance.
- ^ Other early investigators who suspected that the electrical force diminished with distance as the gravitational force did (i.e., as the inverse square of the distance) included Daniel Bernoulli (see: Abel Socin (1760) Acta Helvetiсa, vol. 4, pages 224-225.) and Alessandro Volta, both of whom measured the force between plates of a capacitor, and Aepinus who supposed the inverse-square law in 1758. See: J.L. Heilbron, Electricity in the 17th and 18th Centuries: A Study of Early Modern Physics (Los Angeles, California: University of California Press, 1979), pages 460-462, and 464 (including footnote 44).
- ^ Joseph Priestley, The History and Present State of Electricity, with Original Experiments (London, England: 1767), page 732:
May we not infer from this experiment, that the attraction of electricity is subject to the same laws with that of gravitation, and is therefore according to the squares of the distances; since it is easily demonstrated, that were the earth in the form of a shell, a body in the inside of it would not be attracted to one side more than another?
- ^ Robert S. Elliott (1999). Electromagnetics: History, Theory, and Applications. ISBN 978-0-7803-5384-8. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-0780353846.html
- ^ John Robison, A System of Mechanical Philosophy (London, England: John Murray, 1822), vol. 4. On page 68, the author states that in 1769 he announced his findings regarding the force between spheres of like charge. On page 73, the author states the force between spheres of like charge varies as x-2.06.
- ^ James Clerk Maxwell, ed., The Electrical Researches of the Honourable Henry Cavendish... (Cambridge, England: Cambridge University Press, 1879), pages 104-113: "Experiments on Electricity: Experimental determination of the law of electric force."
- ^ Coulomb's law, Hyperphysics
- ^ Coulomb's constant, Hyperphysics
- ^ Current practice is to use c0 to denote the speed of light in vacuum according to ISO 31. In the original Recommendation of 1983, the symbol c was used for this purpose and continues to be commonly used. See NIST Special Publication 330, Appendix 2, p. 45
- ^ Base unit definitions: Meter. Physics.nist.gov. Retrieved on 2010-09-28.
- ^ Base unit definitions: Ampere. Physics.nist.gov. Retrieved on 2010-09-28.
- ^ CODATA Value: electric constant. Physics.nist.gov. Retrieved on 2010-09-28.
- ^ a b c Coulomb's law, University of Texas
- ^ Charged rods, PhysicsLab.org
References
- Griffiths, David J. (1998). Introduction to Electrodynamics (3rd ed.). Prentice Hall. ISBN 0-13-805326-X.
- Tipler, Paul (2004). Physics for Scientists and Engineers: Electricity, Magnetism, Light, and Elementary Modern Physics (5th ed.). W. H. Freeman. ISBN 0-7167-0810-8.
External links
- Coulomb's Law on Project PHYSNET.
- Electricity and the Atom — a chapter from an online textbook
- A maze game for teaching Coulomb's Law—a game created by the Molecular Workbench software
- Electric Charges, Polarization, Electric Force, Coulomb's Law Walter Lewin, 8.02 Electricity and Magnetism, Spring 2002: Lecture 1 (video). MIT OpenCourseWare. License: Creative Commons Attribution-Noncommercial-Share Alike.
Categories:- Electrostatics
- Introductory physics
- Fundamental physics concepts
Wikimedia Foundation. 2010.