- Phosphorus pentoxide
-
Phosphorus pentoxide 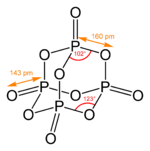
 Other namesDiphosphorus pentoxide
Other namesDiphosphorus pentoxide
Phosphorus(V) oxide
Phosphoric anhydrideIdentifiers CAS number 1314-56-3  ,
,
[16752-60-6] (P4O10)PubChem 14812 ChemSpider 14128 
ChEBI CHEBI:37376 
RTECS number TH3945000 Jmol-3D images Image 1 - O=P13OP2(=O)OP(=O)(O1)OP(=O)(O2)O3
Properties Molecular formula O10P4 Molar mass 283.89 g mol−1 Exact mass 283.889048 g/mol Appearance white powder
very deliquescent
pungent odourDensity 2.39 g/cm3 Melting point 340 °C, 613 K, 644 °F
Boiling point 360 °C (sublimes)
Solubility in water exothermic hydrolysis Vapor pressure 1 mmHg @ 384 °C Hazards MSDS MSDS EU classification not listed NFPA 704  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.
Contents
Structure
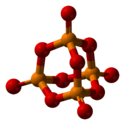
Phosphorus pentoxide crystallizes in at least four forms or polymorphs. The most familiar one, shown in the figure, comprises molecules of P4O10. Weak van der Waals forces hold these molecules together in a hexagonal lattice (However, in spite of the high symmetry of the molecules, the crystal packing is not a close packing[1]). The structure of the P4O10 cage is reminiscent of adamantane with Td symmetry point group.[2] It is closely related to the corresponding anhydride of phosphorous acid, P4O6. The latter lacks terminal oxo groups. Its density is 2.30 g/cm3. It boils at 423 °C under atmospheric pressure; if heated more rapidly it can sublimate.
The other polymorphs are polymeric, but in each case the phosphorus atoms are bound by a tetrahedron of oxygen atoms, one of which forms a terminal P=O bond. The O-form (density 3.05 g/cm3, melting point 580 °C), adopts a layered structure consisting of interconnected P6O6 rings, not unlike the structure adopted by certain polysilicates. A lower density phase, the so-called O' form, consists of a 3-dimensional framework is also known, density 2.72 g/cm3.[3] The remaining polymorph is a glass or amorphous form; it can be made by fusing any of the others.
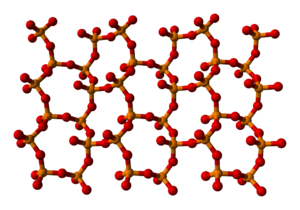
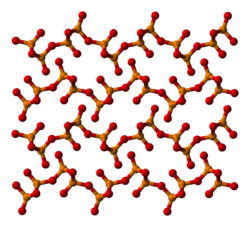
part of an o′-(P2O5)∞ layer o′-(P2O5)∞ layers stacking Preparation
P4O10 is prepared by burning elemental phosphorus with sufficient supply of air:
- P4 + 5 O2 → P4O10
For most of the 20th century, phosphorus pentoxide was used to provide a supply of concentrated pure phosphoric acid. In the thermal process, the phosphorus pentoxide obtained by burning white phosphorus was dissolved in dilute phosphoric acid to produce concentrated acid.[4] Improvements in filter technology is leading to the "wet phosphoric acid process" taking over from the thermal process, obviating the need to produce white phosphorus as a starting material.[5] The dehydration of phosphoric acid to give phosphorus pentoxide is not practicable; on heating, metaphosphoric acid will decompose before it loses water.
Applications
Phosphorus pentoxide is a potent dehydrating agent as indicated by the exothermic nature of its hydrolysis:
- P4O10 + 6 H2O → 4 H3PO4 (–177 kJ)
However, its utility for drying is limited somewhat by its tendency to form a protective viscous coating that inhibits further dehydration by unspent material. A granular form of P4O10 is used in desiccators.
Consistent with its strong desiccating power, P4O10 is used in organic synthesis for dehydration. The most important application is for the conversion of amides into nitriles:[6]
- P4O10 + RC(O)NH2 → P4O9(OH)2 + RCN
The indicated coproduct P4O9(OH)2 is an idealized formula for undefined products resulting from the hydration of P4O10.
Supposedly, when combined with a carboxylic acid, the result is the corresponding anhydride:[7]:
- P4O10 + RCO2H → P4O9(OH)2 + [RC(O)]2O
The "Onodera reagent", a solution of P4O10 in DMSO, is employed for the oxidation of alcohols.[8] This reaction is reminiscent of the Swern oxidation.
The desiccating power of P4O10 is strong enough to convert many mineral acids to their anhydrides. Examples: HNO3 is converted to N2O5; H2SO4 is converted to SO3; HClO4 is converted to Cl2O7.
Related phosphorus oxides
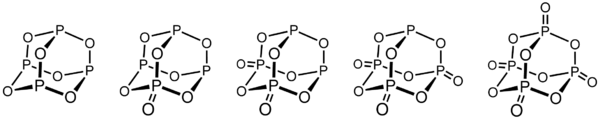
Between the commercially important P4O6 and P4O10, phosphorus oxides are known with intermediate structures.[9]
Hazards
Phosphorus pentoxide is not flammable. It reacts vigorously with water and water-containing substances like wood or cotton, liberates much heat and may even cause fire. It is corrosive to metal and is very irritating – may cause severe burn to the eye, skin, mucous membrane, and respiratory tract even at concentrations as low as 1 mg/m3.[10]
Fiction
- In Anthony Burgess' The Wanting Seed, phosphorus pentoxide is a highly prized compound.[clarification needed]
- In Detective Comics #825, Batman notices that phosphorus pentoxide was at the scene of a fire, indicating that the villain Dr. Phosphorus was involved.
- In Aldous Huxley's Point Counter Point, Lord Edward bemoans societal loss of phosphorous pentoxide to his assistant Illidge.
- In Aldous Huxley's Brave New World, Henry Foster tells Lenina about the recovery of phosphorus pentoxide.
See also
- Eaton's reagent
References
- ^ Cruickshank, D.W.J. (1964). "Refinements of Structures Containing Bonds between Si, P, S or Cl and O or N: V. P4O10". Acta Cryst. 17 (6): 677–9. doi:10.1107/S0365110X64001669.
- ^ D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam. ISBN 0-444-89307-5.
- ^ D. Stachel, I. Svoboda and H. Fuess (June 1995). "Phosphorus Pentoxide at 233 K". Acta Cryst. C51 (6): 1049–1050. doi:10.1107/S0108270194012126.
- ^ Threlfall, Richard E., (1951). The story of 100 years of Phosphorus Making: 1851 - 1951. Oldbury: Albright & Wilson Ltd
- ^ Podger, Hugh (2002). Albright & Wilson: The Last 50 Years. Studley: Brewin Books. ISBN 1-85858-223-7
- ^ Meier, M. S. "Phosphorus(V) Oxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- ^ Joseph C. Salamone, ed (1996). Polymeric materials encyclopedia: C, Volume 2. CRC Press. p. 1417. ISBN 084932470X. http://books.google.com/?id=pzRpQC7CO6sC&pg=PA1417.
- ^ Tidwell, T. T. "Dimethyl Sulfoxide–Phosphorus Pentoxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- ^ Luer, B.; Jansen, M. "Crystal Structure Refinement of Tetraphosphorus Nonaoxide, P4O9" Zeitschrift fur Kristallographie 1991, volume 197, pages 247-8.
- ^ Phosphorus pentoxide MSDS
External links
- OSHA
- Spec sheet
- Definition
- Website of the Technische Universität Darmstadt and the CEEP about Phosphorus Recovery
Phosphorus compounds Categories:- Inorganic phosphorus compounds
- Acid anhydrides
- Acidic oxides
- Desiccants
- Glass compositions
- Dehydrating agents
- Common oxide glass components
Wikimedia Foundation. 2010.