- Phosphorus sesquisulfide
-
P4S3 
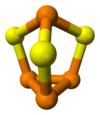
 tetraphosphorus trisulfide
tetraphosphorus trisulfide
or 3,5,7-trithia-1,2,4,6-tetraphosphatricyclo[2.2.1.01,6]heptaneOther namesphosphorus trisulfide, phosphorus sesquisulfide, phosphorus sulfideIdentifiers CAS number 1314-85-8 PubChem 14818 ChemSpider 14134 
RTECS number TH4330000 Jmol-3D images Image 1 - S1P2P3SP1SP23
Properties Molecular formula P4S3 Molar mass 220.093 g/mol Appearance yellow, yellow-green or gray solid Density 2.08 g.cm3,[1] solid Melting point 172.5 °C
Boiling point 408 °C
 (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Phosphorus sesquisulfide is the inorganic compound with the formula P4S3. This yellow solid is one of two commercially produced phosphorus sulfides. It is a component of "strike anywhere" matches.
Depending on purity, samples can appear yellow-green to grey. The compound was discovered by G. Lemoine and first produced safely in commercial quantities in 1898 by Albright and Wilson. It dissolves in an equal weight of carbon disulfide (CS2). Unlike some other phosphorus sulfides, P4S3 is slow to hydrolyze and has a well-defined melting point.
Contents
Structure and synthesis
The molecule has C3v symmetry. It is a derivative of the tetrahedral (P4) unit from insertion of sulfur into three P-P bonds. The P-S and P-P distances are 2.090 and 2.235 Å, respectively. P4Se3 and P4S3 adopt the same structures.[1]
P4S3 is produced by the reaction of red or white phosphorus with sulfur. Excess sulfur gives phosphorus pentasulfide (P4S10). It is estimated that 150 ton/y were produced in 1989.[2]
Applications
A 1:2 mixture of P4S3 and potassium chlorate, together with other materials, comprises the heads of "strike-anywhere matches".[3]
Safety
Its flash point is about 100 °C.
References
- ^ a b Leung, Y. C.; Waser, J.; van Houten, S.; Vos, A.; Wiegers, G. A.; Wiebenga, E. H. “The crystal structure of P4S3”. Acta Crystallographica , Volume 10, pages 574-582, year 1957. DOI:10.1107/S0365110X57002042
- ^ Gerhard Bettermann, Werner Krause, Gerhard Riess, Thomas Hofmann “Phosphorus Compounds, Inorganic” in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a19_527
- ^ D. E. C. Corbridge. Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology. 5th Edition. Elsevier: Amsterdam 1995. ISBN 0-444-89307-5. For a discussion of safety matches, see pages 115-116.
Phosphorus compounds Categories:- Inorganic phosphorus compounds
- Sulfides
Wikimedia Foundation. 2010.
