- Dichlorine heptoxide
-
Dichlorine heptoxide 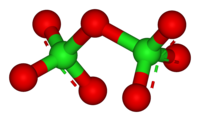 Dichlorine heptoxideOther namesChlorine(VII) oxide; Perchloric anhydride; (Perchloryloxy)chlorane trioxide
Dichlorine heptoxideOther namesChlorine(VII) oxide; Perchloric anhydride; (Perchloryloxy)chlorane trioxideIdentifiers CAS number 10294-48-1 
PubChem 123272 ChemSpider 109884 
ChEBI CHEBI:52356 
Jmol-3D images Image 1 - O=Cl(=O)(=O)OCl(=O)(=O)=O
Properties Molecular formula Cl2O7 Molar mass 182.901 g/mol Appearance colorless oil Density 1900 kg m-3 Melting point −91.5 °C
Boiling point 82 °C
Hazards Main hazards oxidizer, contact explosive[1]  heptoxide (verify) (what is:
heptoxide (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:[1]
- 2 HClO4 + P4O10 → Cl2O7 + H2P4O11
The chlorine(VII) oxide can be distilled off from the mixture.
It may also be formed by illumination on mixtures of chlorine and ozone.[2] It slowly hydrolyzes back to perchloric acid, which is also hazardous when anhydrous.
Contents
Structure
Cl2O7 is an endothermic molecule, which means that it is intrinsically unstable.
- 2 Cl2O7 → 2 Cl2 + 7 O2 (ΔH = -135 kJ/mol)
Cl2O7 is bent with Cl-O-Cl angle of 118.6° giving the molecule C2 symmetry. The terminal Cl-O distances are 1.709 Å and the Cl=O distances are 1.405 Å.[1] In this compound, chlorine exists in its highest formal oxidation state of +7, although the bonding in this molecule is significantly covalent.
Chemistry
Dichlorine heptoxide reacts with primary and secondary amines in carbon tetrachloride solution to yield N-perchloryls:[3]
- 2 RNH2 + Cl2O7 → 2 RNHClO3 + H2O
- 2 R2NH + Cl2O7 → 2 R2NClO3 + H2O
It also reacts with olefins to give alkyl perchlorates. For example, it reacts with propene in carbon tetrachloride solution to yield isopropyl perchlorate and 1-chloro-2-propyl perchlorate.[4]
Safety
Although it is the most stable chlorine oxide, Cl2O7 is a strong oxidizer as well as an explosive that can be set off with flame or mechanical shock, or by contact with iodine.[5] Nevertheless, it is less strongly oxidising than the other chlorine oxides, and does not attack sulfur, phosphorus, or paper when cold.[1] It has the same effects on the human body as elemental chlorine, and requires the same precautions.[6]
References
- ^ a b c d Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Translated by Mary Eagleson, William Brewer. San Diego: Academic Press. p. 464. ISBN 0123526515.
- ^ Byrns, A. C.; Rollefson, G. K. (1934). Journal of the American Chemical Society 56 (5): 1250–1251. doi:10.1021/ja01320a506.
- ^ Beard, C. D.; Baum, K. (1974). "Reactions of dichlorine heptoxide with amines". Journal of the American Chemical Society 96 (10): 3237–3239. doi:10.1021/ja00817a034.
- ^ Baum, K. . (1976). "Reactions of dichlorine heptoxide with olefins". The Journal of Organic Chemistry 41 (9): 1663–1665. doi:10.1021/jo00871a048.
- ^ Lewis, Robert Alan (1998). Lewis' dictionary of toxicology. CRC Press. p. 260. ISBN 1566702232.
- ^ Jeanne Mager Stellman, ed (1998). "Halogens and their compounds". Encyclopaedia of occupational health and safety (4th ed.). International Labour Organization. p. 104.210. ISBN 9221098176.
Chlorine compounds Categories:- Inorganic chlorine compounds
- Acidic oxides
- Oxidizing agents
- Inorganic compound stubs
Wikimedia Foundation. 2010.
