- Piracetam
-
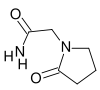
Systematic (IUPAC) name 2-oxo-1-pyrrolidineacetamide Clinical data Trade names Breinox, Dinagen, Lucetam, Nootropil, Nootropyl, Oikamid, and many others AHFS/Drugs.com International Drug Names Pregnancy cat. ? Legal status POM (UK) Routes Oral and parenteral Pharmacokinetic data Bioavailability ~100% Half-life 4 - 5 hr Excretion Urinary Identifiers CAS number 7491-74-9 ATC code N06BX03 PubChem CID 4843 ChemSpider 4677 
UNII ZH516LNZ10 
KEGG D01914 
ChEMBL CHEMBL36715 
Chemical data Formula C6H10N2O2 SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Piracetam (sold under many brand names) is a nootropic drug. Piracetam's chemical name is 2-oxo-1-pyrrolidine acetamide; it shares the same 2-oxo-pyrrolidone base structure with 2-oxo-pyrrolidine carboxylic acid (pyroglutamate). Piracetam is a cyclic derivative of GABA. It is one of the group of racetams. Piracetam is prescribed by doctors for some conditions, mainly myoclonus,[1] but is used off-label for a much wider range of applications.
Popular trade names for Piracetam in Europe are "Nootropil" and "Lucetam", among many others. In South America, it is made by Laboratorios Farma S.A. and sold under the brand name of Breinox in Venezuela and Ecuador.
Contents
Effects
There is very little data on piracetam's effect on healthy people, with most studies focusing on people with seizures, dementia, concussions, or other neurological problems. However, a two week regimen of piracetam was found to enhance verbal memory in healthy college students in a double-blind, placebo-controlled study.[2]
In 2008, a committee of the British Academy of Medical Sciences noted that many of the clinical trials of piracetam for dementia were methodologically flawed.[3] However, numerous positive individual studies support the use of piracetam in people suffering from a wide range of cognitive disorders,[4][5] and a 2002 meta-analysis concluded that piracetam had a therapeutic effect in older patients with cognitive impairment.[6]
Several meta-reviews of literature on piracetam indicate that piracetam increases performance on a variety of cognitive tasks among dyslexic children, though this may reflect its enhancement of cross-hemispheric communication and of cognitive function in general, rather than a specific improvement in whatever causes dyslexia. Piracetam also seems to inhibit brain damage caused by a variety of factors including hypoxia and excessive alcohol consumption.[7][8]
Piracetam has been studied in an extensive number of clinical experiments, and has shown positive results in the treatment of post-stroke aphasia, epilepsy, cognitive decline following heart and brain surgery, dementia,[6] and myoclonus.[9][10] Its peripheral vascular effect has indicated its use for vertigo, dyslexia, and sickle cell anemia as well.[4]
Piracetam possesses pronounced antihypoxic and antiarrhythmic effect; the latter is carried out by decreasing the rhythm rate and increasing the contraction amplitude. The animals treated with piracetam in a dose when its antiarrhythmic effects (300 mg/kg) exhibited a decrease of the membrane potential of erythrocytes as compared with control. Similar effects occurred in the animals treated with lidocaine. It can be concluded that in certain types of arrhythmias the use of piracetam restores the normal rhythm of contractions that is perhaps connected with its positive influence on metabolic processes in the myocardium.[11]
Piracetam appears to increase communication between the two hemispheres of the brain, and increases activity of the corpus callosum.[12][13]
Mechanisms of action
Piracetam's mechanism of action, as with racetams in general, is not fully understood. The drug influences neuronal and vascular functions and influences cognitive function without acting as a sedative or stimulant.[4] Piracetam is a positive allosteric modulator of the AMPA receptor.[14] It is hypothesized to act on ion channels or ion carriers,[citation needed] thus leading to non-specific increased neuron excitability, while explaining its lack of agonistic or inhibitory effect on synaptic action (quite unlike most neurotransmitters), and its low toxicity.[15] GABA brain metabolism and GABA receptors are not affected by piracetam. It has been found to increase blood flow and oxygen consumption in parts of the brain but this may be a side effect of increased brain activity rather than a primary effect or mechanism of action for the drug.[16]
Piracetam improves the function of the neurotransmitter acetylcholine via muscarinic cholinergic (ACh) receptors which are implicated in memory processes.[17] Furthermore, Piracetam may have an effect on NMDA glutamate receptors, which are involved with learning and memory processes. Piracetam is thought to increase cell membrane permeability.[17][18] Piracetam may exert its global effect on brain neurotransmission via modulation of ion channels (i.e., Na+, K+).[15] It has been found to increase oxygen consumption in the brain, apparently in connection to ATP metabolism, and increases the activity of adenylate kinase in rat brains.[19][20] Piracetam appears to increase the synthesis of cytochrome b5,[21] which is a part of the electron transport mechanism in mitochondria. It also increases the permeability of the mitochondria of some intermediaries of the Krebs cycle.[19]
History
Piracetam was first synthesized in 1964 by scientists at the Belgian pharmaceutical company UCB led by Dr Corneliu E. Giurgea; struck by its apparent ability to boost mental functioning in even healthy individuals and by its safety, Giurgea coined the term nootropic to describe it and other substances. Piracetam (trade name "Nootropil") was launched clinically by UCB in the early 1970s, and later is in use in many European countries.[22]
Approval and usage
Piracetam is primarily used in Europe, Asia, South America and the US. Piracetam is legal to import into the United Kingdom for personal use with or without prescription, and was unregulated in the United States, as of 2006.[23] However, in August 2010, the FDA issued a single letter to one supplier ordering the discontinuation of the sale of Piracetam in the US as a dietary supplement owing to the relatively strict definition of what constitutes a dietary supplement. Under section 201(ff)(1) of the Act, 21 U.S.C. § 321(ff)(1): Piracetam is not a vitamin, mineral, amino acid, herb or other botanical, or dietary substance for use by man to supplement the diet by increasing the total dietary intake. Further, piracetam is not a concentrate, metabolite, constituent, extract or combination of any such dietary ingredient.[24] As of April 2011, Piracetam continues to be widely available online and in some shops in the United States, but is no longer marketed as a dietary supplement. Piracetam has no DIN in Canada, and thus cannot be sold but can be imported for personal use in Canada.[citation needed] It has become popular as a cognitive enhancement drug among students.[25] It is used by parents as a treatment for childhood autism,[citation needed] a practice at least partially supported by clinical research.[26]
Aging
Piracetam appears to reverse the effects of aging in the brains of mice.[27][28]
Piracetam appears to reduce levels of lipofuscin in the rat brain.[29] (Lipofuscin accumulation is a common symptom of aging and alcoholism.)
Alcoholism
Piracetam appears to be effective in treating cognitive impairment in alcoholism.[30][31][32][33][34][35]
Alzheimer's and senile dementia
Piracetam appears to be effective for improving cognition in Alzheimer's disease and senile dementia patients,[36][37][38][39][40] although these findings are still challenged.[41]
Clotting, coagulation, vasospastic disorders
Piracetam is useful as a long-term treatment for clotting, coagulation, and vasospastic disorders such as Raynaud's phenomenon[42] and deep-vein thrombosis.[17][43] It is an extremely safe anti-thrombotic agent that operates through the novel mechanism of inhibiting platelet aggregation and enhancing blood-cell deformability.[17] Because traditional anti-thrombotic drugs operate through the separate mechanism of inhibiting clotting factors, co-administration of piracetam has been shown to highly complement the efficacy and safety of traditional Warfarin/Heparin anti-coagulation therapy.[44] The most effective treatment range for this use is a daily dose of 4.8 to 9.6 grams divided into three daily doses at 8 hours apart.[43] Piracetam was investigated as a complement or alternative to Warfarin as a safe and effective long-term treatment for recurring deep-vein thrombosis.[43]
Depression and anxiety
Some sources suggests that its overall effect on lowering depression and anxiety is higher than improving memory.[45]
Stroke, ischemia and symptoms
Piracetam has been found to improve cognition after stroke, and reduce symptoms, such as aphasia.[37] It also improves cognition in cases of chronic ischemia.[46][47]
Dyspraxia and dysgraphia
Due to its supposed effect on nerves and muscles it is sometimes prescribed as an aid to muscle or dexterity training, particularly in cases of dysgraphia and dyspraxia. There has not been a specific study as to whether it is beneficial in this. Vinpocetine, another purported nootropic with which piracetam is indirectly synergistic, is confirmed to help with these conditions to a certain degree.[citation needed]
Schizophrenia
At least one study shows that, while piracetam positively affected the cognition of patients suffering from schizophrenia in the same way as it positively affected the cognition of others, the severity of subjects' schizophrenia remained unaffected, for better or worse.[48]
Preventive for breath-holding spells
Two articles support the use of Piracetam as a prophylactic for severe cases of breath-holding spells. A 2008 study in the International Journal of Psychiatry Medicine supported the notion that Piracetam was effective as a preventive, but did not use a control to evaluate results against normal recovery times from severe BHS.[49] A 1998 study by the Turkish ministry of health evaluated 76 children, half of them in a control group. Children in the experimental group were three times as, and almost completely likely, to exhibit "overall control" over their BHS, with BHS episodes dropping by 60% over two months.[50]
The 2008 study notes:
Breath holding spells (BHS) are apparently frightening events occurring in otherwise healthy children. Generally, no medical treatment is recommended and parental reassurance is believed to be enough, however, severe BHS can be very stressful for the parents and a pharmacological agent may be desired in some of these children.Closed craniocerebral trauma
Piracetam has positive therapeutic effects on adolescents with closed craniocerebral trauma (CCT). Treatment with piracetam was initiated 1.5 to 5 years after trauma. Compared to controls, after one month of daily treatment with 1600–2400 mg of piracetam there were meaningful and statistically significant improvements in the higher mental functions (visual memory, attention and executive), motor functions (gait, balance and sequential limb movements) and in the rates of cognitive and motor operations.[51]
Dosage
For blood coagulation, clotting, and vasospastic disorders such as Raynaud's phenomenon or deep-vein thrombosis, the most effective treatment range is a daily dose of 4.8 to 9.6 grams divided into three daily doses at 8 hours apart.[17][42][43] Many people take a dosage of 800mg twice per day to improve cognition.[52] The LD-50 for oral consumption in humans has not been determined.[15]
Side effects
Piracetam has been found to have very few side effects, and those it has are typically "few, mild, and transient."[53] A large-scale, 12-week trial of high-dose piracetam found no adverse effects occurred in the group taking piracetam as compared to the placebo group.[54] Many other studies have likewise found piracetam to be well-tolerated.[9][53][55]
Symptoms of general excitability, including anxiety, insomnia, irritability, headache, agitation, nervousness, and tremor, are occasionally reported.[56][57] Headache from large doses of piracetam may be alleviated by coadministration of an acetylcholine biosynthetic precursor, or a drug with cholinergic effects, such as choline bitartrate, choline citrate, choline alfoscerate, lecithin, cyprodenate or centrophenoxine.[56][57]
Availability
Piracetam is sold under a wide variety of brand names world wide.
- Nootropyl - United States
- Nootropil, Lucetam, Oikamid - Europe, Brazil
- Breinox - Ecuador, Venezuela
- Dinagen - Mexico
- Noostan - Argentina
Notes
- ^ "Nootropil". NetDoctor.co.uk. 8 July 2004. http://www.netdoctor.co.uk/medicines/100001864.html. Retrieved 21 September 2009.
- ^ Dimond, SJ; Brouwers, EM (1976). "Increase in the power of human memory in normal man through the use of drugs". Psychopharmacology 49 (3): 307–9. doi:10.1007/BF00426834. PMID 826948.
- ^ Talbot, Margaret (27 April 2009). "Brain Gain: The underground world of 'neuroenhancing' drugs". The New Yorker. http://www.newyorker.com/reporting/2009/04/27/090427fa_fact_talbot. Retrieved 21 September 2009.
- ^ a b c Winblad, B (2005). "Piracetam: a review of pharmacological properties and clinical uses". CNS drug reviews 11 (2): 169–82. doi:10.1111/j.1527-3458.2005.tb00268.x. PMID 16007238.
- ^ Flicker, L; Grimley Evans, G; Flicker, Leon (2001). "Piracetam for dementia or cognitive impairment". Cochrane database of systematic reviews (Online) (2): CD001011. doi:10.1002/14651858.CD001011. PMID 11405971.
- ^ a b Waegemans, T; Wilsher, CR; Danniau, A; Ferris, SH; Kurz, A; Winblad, B (2002). "Clinical efficacy of piracetam in cognitive impairment: a meta-analysis". Dementia and geriatric cognitive disorders 13 (4): 217–24. doi:10.1159/000057700. PMID 12006732.
- ^ Vaglenova, J; Vesselinov Petkov, V (2001). "Can nootropic drugs be effective against the impact of ethanol teratogenicity on cognitive performance?". European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 11 (1): 33–40. PMID 11226810.
- ^ Gabryel, B; Adamek, M; Pudełko, A; Małecki, A; Trzeciak, HI (2002). "Piracetam and vinpocetine exert cytoprotective activity and prevent apoptosis of astrocytes in vitro in hypoxia and reoxygenation". Neurotoxicology 23 (1): 19–31. doi:10.1016/S0161-813X(02)00004-9. PMID 12164545.
- ^ a b Fedi, M; Reutens, D; Dubeau, F; Andermann, E; D'agostino, D; Andermann, F (2001). "Long-term efficacy and safety of piracetam in the treatment of progressive myoclonus epilepsy". Archives of neurology 58 (5): 781–6. doi:10.1001/archneur.58.5.781. PMID 11346373.
- ^ Brown, P; Steiger, MJ; Thompson, PD; Rothwell, JC; Day, BL; Salama, M; Waegemans, T; Marsden, CD (1993). "Effectiveness of piracetam in cortical myoclonus". Movement disorders : official journal of the Movement Disorder Society 8 (1): 63–8. doi:10.1002/mds.870080112. PMID 8419809.
- ^ Samvelian, VM; Malakian, MG; Badzhinian, SA (1990). "The antiarrhythmic action of piracetam". Farmakologiia i toksikologiia 53 (6): 22–3. PMID 2081561.
- ^ Giurgea, CE; Moyersoons, FE (1972). "On the pharmacology of cortical evoked potentials". Archives internationales de pharmacodynamie et de therapie 199 (1): 67–78. PMID 4342020.
- ^ Buresová, O; Bures, J (1976). "Piracetam-induced facilitation of interhemispheric transfer of visual information in rats". Psychopharmacologia 46 (1): 93–102. doi:10.1007/BF00421555. PMID 1257371.
- ^ Ahmed, A; Oswald, R (2010). "Piracetam Defines a New Binding Site for Allosteric Modulators of r-Amino-3-hydroxy-5-methyl-4-isoxazole-propionic Acid (AMPA) Receptors". J Med. Chem. 53 (5): 2197–2203. doi:10.1021/jm901905j. PMC 2872987. PMID 20163115. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2872987.
- ^ a b c Gouliaev, AH; Senning, A (1994). "Piracetam and other structurally related nootropics". Brain research. Brain research reviews 19 (2): 180–222. doi:10.1016/0165-0173(94)90011-6. PMID 8061686.
- ^ Jordaan, B; Oliver, DW; Dormehl, IC; Hugo, N (1996). "Cerebral blood flow effects of piracetam, pentifylline, and nicotinic acid in the baboon model compared with the known effect of acetazolamide". Arzneimittel-Forschung 46 (9): 844–7. PMID 8876930.
- ^ a b c d e Winnicka, K; Tomasiak, M; Bielawska, A (2005). "Piracetam--an old drug with novel properties?". Acta poloniae pharmaceutica 62 (5): 405–9. PMID 16459490.
- ^ Müller, WE; Eckert, GP; Eckert, A (1999). "Piracetam: novelty in a unique mode of action". Pharmacopsychiatry 32 Suppl 1: 2–9. doi:10.1055/s-2007-979230. PMID 10338102.
- ^ a b Grau, M; Montero, JL; Balasch, J (1987). "Effect of Piracetam on electrocorticogram and local cerebral glucose utilization in the rat". General pharmacology 18 (2): 205–11. PMID 3569848.
- ^ Nickolson, VJ; Wolthuis, OL (1976). "Effect of the acquisition-enhancing drug piracetam on rat cerebral energy metabolism. Comparison with naftidrofuryl and methamphetamine". Biochemical pharmacology 25 (20): 2241–4. doi:10.1016/0006-2952(76)90004-6. PMID 985556.
- ^ Tacconi, MT; Wurtman, RJ (1986). "Piracetam: physiological disposition and mechanism of action.". Advances in neurology 43: 675–85. PMID 3946121.
- ^ S. D. Shorvon (2004). "Piracetam". In Simon D. Shorvon, David Fish, Emilio Perucca, W E Dodson. The treatment of epilepsy. Wiley–Blackwell. pp. 489–495. ISBN 9780632060467. http://books.google.com/?id=TfrwxdXcmosC&pg=PA489&dq=piracetam+Giurgea#v=onepage&q=piracetam%20Giurgea&f=false.
- ^ http://www.medic8.com/medicines/Piracetam.html
- ^ FDA Warning Letter, August 30, 2010 Retrieved 2011-3-2
- ^ "I would say a lot of students are using that now, he said. Memory enhancers like piracetam are also very popular." http://www.watoday.com.au/national/call-for-testing-on-smart-drugs-20091002-gf8t.html
- ^ Akhondzadeh, S., et al. A double-blind placebo controlled trial of piracetam added to risperidone in patients with autistic disorder. Child Psychiatry and Human Development, Vol 39(3), Sep, 2008. pp. 237-245.
- ^ Pilch, H; Müller, WE (1988). "Piracetam elevates muscarinic cholinergic receptor density in the frontal cortex of aged but not of young mice". Psychopharmacology 94 (1): 74–8. doi:10.1007/BF00735884. PMID 3126530.
- ^ Stoll, L; Schubert, T; Müller, WE (1992). "Age-related deficits of central muscarinic cholinergic receptor function in the mouse: partial restoration by chronic piracetam treatment". Neurobiology of aging 13 (1): 39–44. doi:10.1016/0197-4580(92)90006-J. PMID 1542379.
- ^ Paula-Barbosa, MM; Brandão, F; Pinho, MC; Andrade, JP; Madeira, MD; Cadete-Leite, A (1991). "The effects of piracetam on lipofuscin of the rat cerebellar and hippocampal neurons after long-term alcohol treatment and withdrawal: a quantitative study". Alcoholism, clinical and experimental research 15 (5): 834–8. doi:10.1111/j.1530-0277.1991.tb00610.x. PMID 1755517.
- ^ Skondia, V; Kabes, J (1985). "Piracetam in alcoholic psychoses: a double-blind, crossover, placebo controlled study". The Journal of international medical research 13 (3): 185–7. PMID 3891457.
- ^ Buranji, I; Skocilić, Z; Kozarić-Kovacić, D (1990). "Cognitive function in alcoholics in a double-blind study of piracetam". Lijecnicki vjesnik 112 (3-4): 111–4. PMID 2204773.
- ^ Kalmár, S (2003). "Adjuvant therapy with parenteral piracetam in alcohol withdrawal delirium". Orvosi hetilap 144 (19): 927–30. PMID 12809069.
- ^ Dencker, SJ; Wilhelmson, G; Carlsson, E; Bereen, FJ (1978). "Piracetam and chlormethiazole in acute alcohol withdrawal: a controlled clinical trial". The Journal of international medical research 6 (5): 395–400. PMID 359384.
- ^ Meyer, JG; Forst, R; Meyer-Wahl, L (1979). "Course of alcoholic predelirium during treatment with piracetam: results of serial psychometric tests (author's transl)". Deutsche medizinische Wochenschrift (1946) 104 (25): 911–4. doi:10.1055/s-0028-1104013. PMID 446321.
- ^ Binder, S; Doddabela, P (1976). "The efficacy of Piracetam on the mental functional capacity of chronic alcoholics (author's transl)". Medizinische Klinik 71 (17): 711–6. PMID 775275.
- ^ Croisile, B; Trillet, M; Fondarai, J; Laurent, B; Mauguière, F; Billardon, M (1993). "Long-term and high-dose piracetam treatment of Alzheimer's disease". Neurology 43 (2): 301–5. PMID 8437693.
- ^ a b Deberdt, W (1994). "Interaction between psychological and pharmacological treatment in cognitive impairment". Life sciences 55 (25-26): 2057–66. doi:10.1016/0024-3205(94)00386-6. PMID 7997065.
- ^ Ferns, S. et al. (1982). "Combination choline/Piracetam treatment of senile dementia". Psychopharmacol Bull 18: 96–98.
- ^ Smith, RC; Vroulis, G; Johnson, R; Morgan, R (1984). "Comparison of therapeutic response to long-term treatment with lecithin versus piracetam plus lecithin in patients with Alzheimer's disease". Psychopharmacology bulletin 20 (3): 542–5. PMID 6433396.
- ^ Branconnier, RJ (1983). "The efficacy of the cerebral metabolic enhancers in the treatment of senile dementia". Psychopharmacology bulletin 19 (2): 212–9. PMID 6408682.
- ^ http://onlinelibrary.wiley.com/o/cochrane/clsysrev/articles/CD001011/frame.html
- ^ a b Moriau, M; Lavenne-Pardonge, E; Crasborn, L; Von Frenckell, R; Col-Debeys, C (1993). "Treatment of the Raynaud's phenomenon with piracetam". Arzneimittel-Forschung 43 (5): 526–35. PMID 8328997.
- ^ a b c d Moriau, M; Crasborn, L; Lavenne-Pardonge, E; Von Frenckell, R; Col-Debeys, C (1993). "Platelet anti-aggregant and rheological properties of piracetam. A pharmacodynamic study in normal subjects". Arzneimittel-Forschung 43 (2): 110–8. PMID 8457235.
- ^ Moriau, M; Lavenne-Pardonge, E; Crasborn, L; Von Frenckell, R; Col-Debeys, C (1995). "The treatment of severe or recurrent deep venous thrombosis. Beneficial effect of the co-administration of antiplatelet agents with or without rheological effects, and anticoagulants". Thrombosis research 78 (6): 469–82. doi:10.1016/0049-3848(95)00081-2. PMID 15714749.
- ^ Malykh AG, Sadaie MR (February 2010). "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders". Drugs 70 (3): 287–312. doi:10.2165/11319230-000000000-00000. PMID 20166767.
- ^ Herrschaft, H. (1989). "Effects and therapeutic efficacy of nootropic drugs in acute and chronic cerebral ischaemia in man". In Josef Krieglstein. Pharmacology of Cerebral Ischemia. Boca Raton: CRC Press. pp. 481–. ISBN 978-3-8047-1036-8. OCLC 20898140.[page needed]
- ^ Platt, D; Horn, J; Summa, JD; Schmitt-Rüth, R; Kauntz, J; Krönert, E (1993). "On the efficacy of piracetam in geriatric patients with acute cerebral ischemia: a clinically controlled double-blind study". Archives of gerontology and geriatrics 16 (2): 149–64. doi:10.1016/0167-4943(93)90006-4. PMID 15374345.
- ^ Dimond, Stuart J.; Scammell, RE; Pryce, IG; Huws, D; Gray, C (1979). "Some effects of piracetam (UCB 6215 nootropyl) on chronic schizophrenia". Psychopharmacology 64 (3): 341–8. doi:10.1007/BF00427522. PMID 116278.
- ^ Azam M, Bhatti N, Shahab N (2008). "Piracetam in severe breath holding spells". Int J Psychiatry Med 38 (2): 195–201. doi:10.2190/PM.38.2.f. PMID 18724570.
- ^ Donma MM (January 1998). "Clinical efficacy of piracetam in treatment of breath-holding spells". Pediatr. Neurol. 18 (1): 41–5. doi:10.1016/S0887-8994(97)00153-7. PMID 9492090.
- ^ Zavadenko, NN; Guzilova, LS (2009). "Sequelae of closed craniocerebral trauma and the efficacy of piracetam in its treatment in adolescents". Neuroscience and behavioral physiology 39 (4): 323–8. doi:10.1007/s11055-009-9146-2. PMID 19340573.
- ^ [1]
- ^ a b Koskiniemi, M; Van Vleymen, B; Hakamies, L; Lamusuo, S; Taalas, J (1998). "Piracetam relieves symptoms in progressive myoclonus epilepsy: a multicentre, randomised, double blind, crossover study comparing the efficacy and safety of three dosages of oral piracetam with placebo". Journal of neurology, neurosurgery, and psychiatry 64 (3): 344–8. doi:10.1136/jnnp.64.3.344. PMC 2169975. PMID 9527146. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2169975.
- ^ De Reuck, J; Van Vleymen, B (1999). "The clinical safety of high-dose piracetam--its use in the treatment of acute stroke". Pharmacopsychiatry 32 Suppl 1: 33–7. doi:10.1055/s-2007-979234. PMID 10338106.
- ^ Giurgea, C.; Salama, M. (1977). "Nootropic drugs". Prog Neuro-Psychopharmacolgy 1: 235–247. doi:10.1016/0364-7722(77)90046-7.
- ^ a b Chouinard, G; Annable, L; Ross-Chouinard, A; Olivier, M; Fontaine, F (1983). "Piracetam in elderly psychiatric patients with mild diffuse cerebral impairment". Psychopharmacology 81 (2): 100–6. doi:10.1007/BF00429000. PMID 6415738.
- ^ a b Hakkarainen, H; Hakamies, L (1978). "Piracetam in the treatment of post-concussional syndrome. A double-blind study". European neurology 17 (1): 50–5. doi:10.1159/000114922. PMID 342247.
See also
- Hydergine
- Levetiracetam - an analogue of Piracetam bearing an additional CH3-CH2- sidechain and bearing antiepileptic pharmacological properties through an poorly understood mechanism probably related to it's affinity for the vesicle protein SV2A
- Brivaracetam - another analogue of Piracetam with the same additional side chain as Levetiracetam and a three carbon chain. It exibits greater antiepileptic properties than Levetiracetam but with a somewhat smaller, although still high, theraputic range. As of 2011, it is in 3rd stage pharmaceutical trials
References
- UCB Pharma Limited (2005). "Nootropil 800 mg & 1200 mg Tablets and Solution". electronic Medicines Compendium. Datapharm Communications. http://emc.medicines.org.uk/emc/assets/c/html/displaydoc.asp?documentid=16509. Retrieved 8 December 2005.
External links
- Erowid Piracetam Vault
- Erowid Piracetam FAQ
- Collection of Scientific Abstracts on Piracetam
- Piracetam (Nootropyl) by Ward Dean, M.D., and John Morgenthaler
Racetams Aloracetam • Aniracetam • Brivaracetam • Cebaracetam • Coluracetam • Dimiracetam • Doliracetam • Dupracetam • Etiracetam/Levetiracetam • Fasoracetam • Imuracetam • Molracetam • Nebracetam • Nefiracetam • Noopept • Oxiracetam • Phenylpiracetam • Piperacetam • Piracetam • Pramiracetam • Rolipram • Rolziracetam • SeletracetamPsychostimulants, agents used for ADHD, and nootropics (N06B) Centrally acting sympathomimetics Xanthine derivatives Glutamate receptor CX-516 • CX-546 • CX-614 • CX-691 • CX-717 • IDRA-21 • LY-404,187 • LY-503,430 • PEPA • S-18986 • Sunifiram • UnifiramEugeroics / Benzhydryl compounds Histamine H3 receptor antagonists GABAA α5 inverse agonists Dopamine D1 receptor agonists α7 nicotinic agonists / PAMs AR-R17779 • PNU-282,987 • SSR-180,711Prolyl endopeptidase inhibitors S-17092Alpha-adrenergic agonists Other psychostimulants and nootropics Acetylcarnitine • Adafenoxate • Bifemelane • Carbenoxolone • Citicoline • Cyprodenate • Ensaculin • Idebenone • Ispronicline • Deanol • Dimebon • Fipexide • Leteprinim • Linopirdine • Meclofenoxate • Nizofenone • P7C3 • Pirisudanol • Pyritinol • Rubidium • Sulbutiamine • Taltirelin • Tricyanoaminopropene • VinpocetineCategories:- Racetams
- Pyrrolidones
- AMPA receptor modulators
- Acetamides
Wikimedia Foundation. 2010.
