- Ergoloid
-
Ergoloid 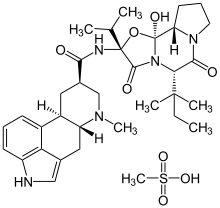
Systematic (IUPAC) name (6aR,9R)-N-((2S,5S,10aS,10bR)-10b-hydroxy-2-isopropyl-3,6-dioxo-5-tert-pentyloctahydro-2H- Clinical data Pregnancy cat. ? Legal status ? Routes Oral, Parenteral Pharmacokinetic data Bioavailability 25% Protein binding 98-99% Metabolism 50% Half-life 3.5 hours Identifiers CAS number 8067-24-1 
ATC code C04AE01 PubChem CID 71171 DrugBank APRD00711 ChemSpider 64311 
ChEBI CHEBI:59756 
ChEMBL CHEMBL1201238 
Chemical data Formula C33H45N5O5 Mol. mass 591.741 g/mol SMILES eMolecules & PubChem - InChI=1S/C33H37N5O5.CH4O3S/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32;1-5(2,3)4/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39);1H3,(H,2,3,4)/t21-,23-,25-,26+,27+,32-,33+;/m1./s1

Key:ADYPXRFPBQGGAH-UMYZUSPBSA-N
 (what is this?) (verify)
(what is this?) (verify)Ergoloid mesylates (USAN) or codergocrine mesilate (BAN), trade name Hydergine, is a mixture of the methanesulfonate salts of three dihydrogenated ergot alkaloids (dihydroergocristine, dihydroergocornine, and alpha- and beta-dihydroergocryptine).
It was developed by Albert Hofmann (the same discoverer of LSD) for Sandoz (now part of Novartis).
Contents
Uses
It has been used to treat dementia and age-related cognitive impairment (such as in Alzheimer disease),[1] as well as to aid in recovery after stroke.
Ergoloids are also used by many people as a nootropic.
Mechanism of action
Despite the fact that hydergine has been used in the treatment of dementia for many years, its mechanism of action is still not clear. It stimulates dopaminergic and serotonergic receptors and blocks alpha-adrenoreceptors.[2] Current studies imply that the major effect of hydergine may be the modulation of synaptic neurotransmission rather than solely increasing blood flow as was once thought. A prominent feature that accompanies aging is an increase in monoamine oxidase (MAO) levels which results in decreased availability of catecholamines in the synaptic cleft. In one study, an interaction between age and hydergine treatment was observed in the hypothalamus, hippocampus and cerebellum. The hydergine effect was more pronounced in the aged group in the hypothalamus and cerebellum, and more pronounced in the adult in the hippocampus. These findings imply that increased brain MAO activity in aging can be modified by hydergine treatment in some brain regions.
Names
Brand names
Hydergine, Hydergina, Gerimal, Niloric, Redizork, Alkergot, Cicanol, Redergin.
Chemical agent synonyms and analogues
Co-Dergocrine Mesylate, Deapril-ST, Dihydroergotoxin Mesilat, Dihydroergotoxin Mesylate, Dihydroergotoxin Methanesulfonate, Dihydroergotoxine Mesilate, Dihydroergotoxine Mesylate, Dihydroergotoxine Methanesulfonate, Dihydroergotoxine Methanesulphonate, Ergoloid Mesylates, Hydergine, Hydergine LC, Hydrogenated Ergot Alkaloids, Ischelium.
Contra-indications
Hydergine (ergoloid mesylates) preparations are contraindicated in individuals who have previously shown hypersensitivity to the drug. Hydergine (ergoloid mesylates) preparations are also contraindicated in patients who have psychosis, acute or chronic, regardless of etiology. Specific drug interactions are unknown so administration should be done with care.
Precautions
Pleural and peritoneal fibrosis have been reported with prolonged daily use. Cardiac valvular fibrosis has also been associated with ergot alkaloids.[citation needed]
Adverse reactions
Adverse effects are minimal. The most common include transient, dose dependent nausea and gastrointestinal disturbances and sublingual irritation with SL tablets. Other common side effects include:
Cardiovascular: orthostatic hypotension, bradycardia
Dermatologic: skin rash, flushing
Ocular: blurred vision
Respiratory: nasal congestion
Dosage
1 mg three times per day is the recommended dosage. Alleviation of symptoms is usually gradual and effects may be seen in 3–4 weeks. It may be used in conjunction with other cerebral enhancers like Piracetam, with which it is synergistic. [3]
Sizes: 4.5 mg p/T (x30) or 1.5 mg p/T
There is some evidence suggesting that potentially effective doses may be higher than those currently approved.[4]
References
- ^ Flynn BL, Ranno AE (February 1999). "Pharmacologic management of Alzheimer disease, Part II: Antioxidants, antihypertensives, and ergoloid derivatives". Ann Pharmacother 33 (2): 188–97. doi:10.1345/aph.17172. PMID 10084415. http://www.theannals.com/cgi/pmidlookup?view=long&pmid=10084415.
- ^ Markstein R (1985). "Hydergine: interaction with the neurotransmitter systems in the central nervous system.". J Pharmacol. 16 Suppl 3: 1–17. PMID 2869188.
- ^ Life Extension Durk Pearson and Sandy Shaw. Warner Books 1983, Part III Chapter 2 Revitalizing Your Brain Power
- ^ Schneider, LS; Olin (August 1994). "Overview of clinical trials of hydergine in dementia". Archives of Neurology 51 (8): 787–98. PMID 8042927.
External links
Ergolines Lysergic acid derivatives 2-Bromo-LSD (BOL-148) • Bromocriptine • Cabergoline • Dihydroergocornine • Dihydroergocristine • Dihydroergocryptine • Dihydroergometrine (Dihydroergonovine, Dihydroergobasine) • Dihydroergotamine • Dihydroergotoxine • Ergine (LSA; LA-111; Lysergamide) • Ergocornine • Ergocristine • Ergocryptine • Ergoloid • Ergometrine (Ergonovine, Ergobasine) • Ergometrinine • Ergotamine • Ergotoxine • Ergovaline • Lisuride • LSD • LSH • Lysergic Acid • Lysergic acid cyclobutylamide • Lysergic acid cyclopentylamide • Lysergic Acid Methyl Ester • Lysergol • Mesulergine • Metergoline • Methergine (Methylergometrine, Methylergonovine, Methylergobasine) • Methysergide • Pergolide • SyntometrinePsychedelic lysergamides AL-LAD • ALD-52 • BU-LAD • CYP-LAD • DAL • DAM-57 • Ergonovine • ETH-LAD • IP-LAD • LAE-32 • LSD • LPD-824 • LSM-775 • LSH • LSD-Pip • Lysergic Acid 2-Butylamide • Lysergic Acid 2,4-Dimethylazetidide • Lysergic Acid 3-Pentylamide • Methylergonovine • Methylisopropyllysergamide • MLD-41 • PARGY-LAD • PRO-LADOther ergolines Natural sources Achnatherum robustum (Sleepy Grass) • Argyreia nervosa (Hawaiian Baby Woodrose) • Claviceps spp. (Ergot) • Ipomoea spp. (Morning Glory, Tlitliltzin, Badoh Negro) • Rivea corymbosa (Coaxihuitl, Ololiúqui)Peripheral vasodilators (C04) 2-amino-1-phenylethanol derivatives Isoxsuprine • Buphenine • BamethanImidazoline derivatives/
Alpha blockersNiacin and derivatives Purine derivatives Pentifylline • Xantinol nicotinate • Pentoxifylline • Etofylline nicotinateErgot alkaloids Ergoloid • Nicergoline • DihydroergocristineOther peripheral vasodilators Cyclandelate • Phenoxybenzamine • Vincamine • Moxisylyte • Bencyclane • Vinburnine • Sulcotidil • Buflomedil • Naftidrofuryl • Butalamine • Visnadine • Cetiedil • Cinepazide • Ifenprodil • Azapetine • FasudilCategories:- Nootropics
- Oxazolopyrrolopyrazines
- Lysergamides
- Lactams
- InChI=1S/C33H37N5O5.CH4O3S/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32;1-5(2,3)4/h3-6,8-11,17,21,23,25-27,34,42H,7,12-16,18H2,1-2H3,(H,35,39);1H3,(H,2,3,4)/t21-,23-,25-,26+,27+,32-,33+;/m1./s1
Wikimedia Foundation. 2010.
