- Acetylcholine
-
Acetylcholine 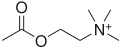
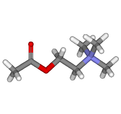 2-Acetoxy-N,N,N-trimethylethanaminium[citation needed]
2-Acetoxy-N,N,N-trimethylethanaminium[citation needed]Identifiers Abbreviations ACh CAS number 51-84-3 
PubChem 187 ChemSpider 182 
UNII N9YNS0M02X 
EC number 200-128-9 DrugBank EXPT00412 KEGG C01996 
MeSH Acetylcholine ChEBI CHEBI:15355 ChEMBL CHEMBL667 
IUPHAR ligand 294 Beilstein Reference 1764436 Gmelin Reference 326108 3DMet B00379 Jmol-3D images Image 1 - CC(=O)OCC[N+](C)(C)C
Properties Molecular formula C7NH16O+
2Molar mass 146.2074 g mol-1 Exact mass 146.118103761 g mol-1 Pharmacology Elimination
half-life2 min  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references The chemical compound acetylcholine (often abbreviated ACh) is a neurotransmitter in both the peripheral nervous system (PNS) and central nervous system (CNS) in many organisms including humans. Acetylcholine is one of many neurotransmitters in the autonomic nervous system (ANS) and the only neurotransmitter used in the motor division of the somatic nervous system (sensory neurons use glutamate and various peptides at their synapses). Acetylcholine is also the principal neurotransmitter in all autonomic ganglia.
Acetylcholine slows the heart rate when functioning as an inhibitory neurotransmitter. However, acetylcholine also behaves as an excitatory neurotransmitter at neuromuscular junctions.[1]
Contents
History
Acetylcholine (ACh) was first identified in 1914 by Henry Hallett Dale for its actions on heart tissue. It was confirmed as a neurotransmitter by Otto Loewi, who initially gave it the name Vagusstoff because it was released from the vagus nerve. Both received the 1936 Nobel Prize in Physiology or Medicine for their work. Acetylcholine was also the first neurotransmitter to be identified.
Chemistry
Acetylcholine is an ester of acetic acid and choline with chemical formula CH3COOCH2CH2N+(CH3)3. This structure is reflected in the systematic name, 2-acetoxy-N,N,N-trimethylethanaminium. Its receptors have very high binding constants.
Function
Acetylcholine Abbreviation ACh Sources many Targets many Receptors nicotinic; muscarinic Agonists nicotine, physostigmine Antagonists curare, atropine Precursor choline Synthesizing enzyme Choline acetyltransferase (ChAT) Metabolizing enzyme Acetylcholinesterase (AChE) Acetylcholine has functions both in the peripheral nervous system (PNS) and in the central nervous system (CNS) as a neuromodulator.
In the peripheral nervous system, acetylcholine activates muscles, and is a major neurotransmitter in the autonomic nervous system.
In the central nervous system, acetylcholine and the associated neurons form a neurotransmitter system, the cholinergic system, which tends to cause anti-excitatory actions.
In the peripheral nervous system
In the peripheral nervous system, acetylcholine activates muscles, and is a major neurotransmitter in the autonomic nervous system. When acetylcholine binds to acetylcholine receptors on skeletal muscle fibers, it opens ligand-gated sodium channels in the cell membrane. Sodium ions then enter the muscle cell, initiating a sequence of steps that finally produce muscle contraction. Although acetylcholine induces contraction of skeletal muscle, it acts via a different type of receptor (muscarinic) to inhibit contraction of cardiac muscle fibers.
In the autonomic nervous system, acetylcholine is released in the following sites:
- all pre- and post-ganglionic parasympathetic neurons
- all preganglionic sympathetic neurons
- preganglionic sympathetic fibers to suprarenal medulla, the modified sympathetic ganglion; on stimulation by acetylcholine, the suprarenal medulla releases epinephrine and norepinephrine
- some postganglionic sympathetic fibers
- pseudomotor neurons to sweat glands.
In the central nervous system
 Micrograph of the nucleus basalis (of Meynert), which produces acetylcholine in the CNS. LFB-HE stain.
Micrograph of the nucleus basalis (of Meynert), which produces acetylcholine in the CNS. LFB-HE stain.
In the central nervous system, ACh has a variety of effects as a neuromodulator upon plasticity, arousal and reward. ACh has an important role in the enhancement of sensory perceptions when we wake up[2] and in sustaining attention.[3]
Damage to the cholinergic (acetylcholine-producing) system in the brain has been shown to be plausibly associated with the memory deficits associated with Alzheimer's disease.[4] ACh has also been shown to promote REM sleep[5]
Pathways
There are three ACh pathways in the CNS.[citation needed]
- Pons to thalamus and cortex
- Magnocellular forebrain nucleus to cortex
- Septohippocampal
Structure
Acetylcholine and the associated neurons form a neurotransmitter system, the cholinergic system from the brainstem and basal forebrain that projects axons to many areas of the brain. In the brainstem it originates from the Pedunculopontine nucleus and dorsolateral tegmental nuclei collectively known as the mesopontine tegmentum area or pontomesencephalotegmental complex.[6][7] In the basal forebrain, it originates from the basal optic nucleus of Meynert and medial septal nucleus:
- The pontomesencephalotegmental complex acts mainly on M1 receptors in the brainstem, deep cerebellar nuclei, pontine nuclei, locus ceruleus, raphe nucleus, lateral reticular nucleus and inferior olive.[7] It also projects to the thalamus, tectum, basal ganglia and basal forebrain.[6]
- Basal optic nucleus of Meynert acts mainly on M1 receptors in the neocortex.
- Medial septal nucleus acts mainly on M1 receptors in the hippocampus and neocortex.
In addition, ACh acts as an important "internal" transmitter in the striatum, which is part of the basal ganglia. It is released by a large set of interneurons with smooth dendrites, known as tonically active neurons or TANs.
Plasticity
ACh is involved with synaptic plasticity, specifically in learning and short-term memory.
Acetylcholine has been shown to enhance the amplitude of synaptic potentials following long-term potentiation in many regions, including the dentate gyrus, CA1, piriform cortex, and neocortex. This effect most likely occurs either through enhancing currents through NMDA receptors or indirectly by suppressing adaptation. The suppression of adaptation has been shown in brain slices of regions CA1, cingulate cortex, and piriform cortex, as well as in vivo in cat somatosensory and motor cortex by decreasing the conductance of voltage-dependent M currents and Ca2+-dependent K+ currents.[citation needed]
Excitability and inhibition
Acetylcholine also has other effects on neurons. One effect is to cause a slow depolarization[citation needed] by blocking a tonically-active K+ current, which increases neuronal excitability. Alternatively, acetylcholine can activate non-specific cation conductances to directly excite neurons.[8] An effect upon postsynaptic M4-muscarinic ACh receptors is to open inward-rectifier potassium ion channel (Kir) and cause inhibition.[9] The influence of acetylcholine on specific neuron types can be dependent upon the duration of cholinergic stimulation. For instance, transient exposure to acetylcholine (up to several seconds) can inhibit cortical pyramidal neurons via M1 type muscarinic receptors that are linked to Gq-type G-protein alpha subunits. M1 receptor activation can induce calcium-release from intracellular stores, which then activate a calcium-activated potassium conductance which inhibits pyramidal neuron firing.[10] On the other hand, tonic M1 receptor activation is strongly excitatory. Thus, ACh acting at one type of receptor can have multiple effects on the same postsynaptic neuron, depending on the duration of receptor activation.[11] Recent experiments in behaving animals have demonstrated that cortical neurons indeed experience both transient and persistent changes in local acetylcholine levels during cue-detection behaviors.[12]
In the cerebral cortex, tonic ACh inhibits layer 4 medium spiny neurons, the main targets of thalamocortical inputs while exciting pyramidal cells in layers 2/3 and layer 5.[9] This filters out weak sensory inputs in layer 4 and amplifies inputs that reach the layers 2/3 and layer L5 excitatory microcircuits. As a result, these layer-specific effects of ACh might function to improve the signal noise ratio of cortical processing.[9] At the same time, acetylcholine acts through nicotinic receptors to excite certain groups of inhibitory interneurons in the cortex, which further dampen down cortical activity.[13]
Another theory[citation needed] interprets acetylcholine neuromodulation in the neocortex as modulating the estimate of expected uncertainty, acting counter to norepinephrine (NE) signals for unexpected uncertainty. Both modulations would then decrease synaptic transition strength, but ACh would then be needed to counter the effects of NE in learning, a signal understood to be 'noisy'.
Synthesis and degradation
Acetylcholine is synthesized in certain neurons by the enzyme choline acetyltransferase from the compounds choline and acetyl-CoA.
The enzyme acetylcholinesterase converts acetylcholine into the inactive metabolites choline and acetate. This enzyme is abundant in the synaptic cleft, and its role in rapidly clearing free acetylcholine from the synapse is essential for proper muscle function. Certain neurotoxins work by inhibiting acetylcholinesterase, thus leading to excess acetylcholine at the neuromuscular junction, thus causing paralysis of the muscles needed for breathing and stopping the beating of the heart.
Receptors
Main article: Acetylcholine receptorThere are two main classes of acetylcholine receptor (AChR), nicotinic acetylcholine receptors (nAChR) and muscarinic acetylcholine receptors (mAChR). They are named for the ligands used to activate the receptors.
Nicotinic
Nicotinic AChRs are ionotropic receptors permeable to sodium, potassium, and chloride ions. They are stimulated by nicotine and acetylcholine. They are of two main types, muscle type and neuronal type. The former can be selectively blocked by curare and the latter by hexamethonium. The main location of nicotinic AChRs is on muscle end plates, autonomic ganglia (both sympathetic and parasympathetic), and in the CNS.[14]
Myasthenia gravis
The disease myasthenia gravis, characterized by muscle weakness and fatigue, occurs when the body inappropriately produces antibodies against acetylcholine nicotinic receptors, and thus inhibits proper acetylcholine signal transmission. Over time, the motor end plate is destroyed. Drugs that competitively inhibit acetylcholinesterase (e.g., neostigmine, physostigmine, or primarily pyridostigmine) are effective in treating this disorder. They allow endogenously-released acetylcholine more time to interact with its respective receptor before being inactivated by acetylcholinesterase in the gap junction.
Muscarinic
Muscarinic receptors are metabotropic, and affect neurons over a longer time frame. They are stimulated by muscarine and acetylcholine, and blocked by atropine. Muscarinic receptors are found in both the central nervous system and the peripheral nervous system, in heart, lungs, upper GI tract and sweat glands. Extracts from the plant Deadly nightshade included this compound (atropine), and the blocking of the muscarinic AChRs increases pupil size as used for attractiveness in many European cultures in the past. Now, ACh is sometimes used during cataract surgery to produce rapid constriction of the pupil. It must be administered intraocularly because corneal cholinesterase metabolizes topically-administered ACh before it can diffuse into the eye. It is sold by the trade name Miochol-E (CIBA Vision). Similar drugs are used to induce mydriasis (dilation of the pupil), in cardiopulmonary resuscitation and many other situations.
Drugs acting on the acetylcholine system
Blocking, hindering or mimicking the action of acetylcholine has many uses in medicine. Drugs acting on the acetylcholine system are either agonists to the receptors, stimulating the system, or antagonists, inhibiting it.
ACh receptor agonists/antagonists
Acetylcholine receptor agonists and antagonists can either have an effect directly on the receptors or exert their effects indirectly, e.g., by affecting the enzyme acetylcholinesterase, which degrades the receptor ligand. Agonists increase the level of receptor activation, antagonists reduce it.
Associated disorders
ACh Receptor Agonists are used to treat myasthenia gravis and Alzheimer's disease.
Alzheimer's disease
Since α4β2 AchRs are reduced in Alzheimer's disease, drugs that inhibit acetylcholinesterase, e.g. galanthamine (a competitive and reversible cholinesterase inhibitor), are commonly used in the treatment of that disease.
Direct acting
These are drugs that mimic acetylcholine on the receptor. In low doses, they stimulate the receptors, in high they numb them due to depolarisation block.
- Acetyl l-carnitine[15]
- Acetylcholine itself
- Bethanechol
- Carbachol
- Cevimeline
- Muscarine
- Nicotine
- Pilocarpine
- Suberylcholine
- Suxamethonium
Cholinesterase inhibitors
Main article: Cholinesterase inhibitorsMost indirect acting ACh receptor agonists work by inhibiting the enzyme acetylcholinesterase. The resulting accumulation of acetylcholine causes continuous stimulation of the muscles, glands, and central nervous system.
They are examples of enzyme inhibitors, and increase the action of acetylcholine by delaying its degradation; some have been used as nerve agents (Sarin and VX nerve gas) or pesticides (organophosphates and the carbamates). In clinical use, they are administered to reverse the action of muscle relaxants, to treat myasthenia gravis, and to treat symptoms of Alzheimer's disease (rivastigmine, which increases cholinergic activity in the brain).
Reversible
The following substances reversibly inhibit the enzyme acetylcholinesterase (which breaks down acetylcholine), thereby increasing acetylcholine levels.
- Many medications in Alzheimer's disease
- Edrophonium (differs myasthenic and cholinergic crisis)
- Neostigmine (commonly used to reverse the effect of neuromuscular blockers used in anaesthesia, or less often in myasthenia gravis)
- Physostigmine (in glaucoma and anticholinergic drug overdoses)
- Pyridostigmine (in myasthenia gravis
- Carbamate insecticides (e.g., Aldicarb)
- Huperzine A
Irreversible
Semi-permanently inhibit the enzyme acetylcholinesterase.
- Echothiophate
- Isofluorophate
- Organophosphate Insecticides (Malathion, Parathion, Azinphos methyl, Chlorpyrifos, among others)
- Organophosphate-containing nerve agents (e.g., Sarin, VX)
Victims of organophosphate-containing nerve agents commonly die of suffocation as they cannot relax their diaphragm.
Reactivation of acetylcholine esterase
ACh receptor antagonists
Antimuscarinic agents
Ganglionic blockers
- Mecamylamine
- Hexamethonium
- Nicotine (in high doses)
- Trimethaphan
Neuromuscular blockers
- Atracurium
- Cisatracurium
- Doxacurium
- Metocurine
- Mivacurium
- Pancuronium
- Rocuronium
- Succinylcholine
- Tubocurarine
- Vecuronium
- Hemicholine
Synthesis inhibitors
- Organic mercurial compounds, such as methylmercury, have a high affinity for sulfhydryl groups, which causes dysfunction of the enzyme choline acetyltransferase. This inhibition may lead to acetylcholine deficiency, and can have consequences on motor function.
Release inhibitors
- Botulin acts by suppressing the release of acetylcholine; where the venom from a black widow spider (alpha-latrotoxin) has the reverse effect.
Other/uncategorized/unknown
References
- ^ Campbell, N. A.; Reece, J. B. (2002). "48". Biology (6th ed.). San Francisco, CA: Pearson Education, Inc.. pp. 1037. ISBN 0-8053-6624-5.
- ^ Jones, BE (2005). "From waking to sleeping: neuronal and chemical substrates". Trends in pharmacological sciences 26 (11): 578–86. doi:10.1016/j.tips.2005.09.009. PMID 16183137.
- ^ Himmelheber, AM; Sarter, M; Bruno, JP (2000). "Increases in cortical acetylcholine release during sustained attention performance in rats". Brain research. Cognitive brain research 9 (3): 313–25. doi:10.1016/S0926-6410(00)00012-4. PMID 10808142.
- ^ http://www.biopsychiatry.com/alzheim.htm
- ^ Platt, Bettina; Riedel, Gernot (2011). "The cholinergic system, EEG and sleep". Behavioural Brain Research 221 (2): 499–504. doi:10.1016/j.bbr.2011.01.017. PMID 21238497.
- ^ a b Woolf, NJ; Butcher, LL (1986). "Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain". Brain research bulletin 16 (5): 603–37. doi:10.1016/0361-9230(86)90134-6. PMID 3742247.
- ^ a b Woolf, NJ; Butcher, LL (1989). "Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum". Brain research bulletin 23 (6): 519–40. doi:10.1016/0361-9230(89)90197-4. PMID 2611694.
- ^ Haj-Dahmane, S; Andrade, R (1996). "Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex". Journal of Neuroscience 16 (12): 3848–61. PMID 8656279.
- ^ a b c Eggermann, E; Feldmeyer, D (2009). "Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4". Proceedings of the National Academy of Sciences of the United States of America 106 (28): 11753–8. doi:10.1073/pnas.0810062106. PMC 2710689. PMID 19564614. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2710689.
- ^ Gulledge, AT; Stuart, GJ (2005). "Cholinergic inhibition of neocortical pyramidal neurons". Journal of Neuroscience 25 (44): 10308–20. doi:10.1523/JNEUROSCI.2697-05.2005. PMID 16267239.
- ^ Gulledge, AT; Bucci, DJ; Zhang, SS; Matsui, M; Yeh, HH (2009). "M1 Receptors Mediate Cholinergic Modulation of Excitability in Neocortical Pyramidal Neurons". Journal of Neuroscience 29 (31): 9888–902. doi:10.1523/JNEUROSCI.1366-09.2009. PMC 2745329. PMID 19657040. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2745329.
- ^ Parikh, V; Kozak, R; Martinez, V; Sarter, M (2007). "Prefrontal acetylcholine release controls cue detection on multiple time scales". Neuron 56 (1): 141–54. doi:10.1016/j.neuron.2007.08.025. PMC 2084212. PMID 17920021. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2084212.
- ^ Gulledge, AT; Park, SB; Kawaguchi, Y; Stuart, GJ (2007). "Heterogeneity of phasic cholinergic signaling in neocortical neurons". Journal of neurophysiology 97 (3): 2215–29. doi:10.1152/jn.00493.2006. PMID 17122323.
- ^ Katzung, B.G. (2003). Basic and Clinical Pharmacology (9th ed.). McGraw-Hill Medical. ISBN 0-07-141092-9
- ^ Nałecz, Ka; Miecz, D; Berezowski, V; Cecchelli, R (Oct 2004). "Carnitine: transport and physiological functions in the brain". Molecular aspects of medicine 25 (5–6): 551–67. doi:10.1016/j.mam.2004.06.001. ISSN 0098-2997. PMID 15363641.
- Brenner, G. M. and Stevens, C. W. (2006). Pharmacology (2nd ed.). Philadelphia, PA: W.B. Saunders Company (Elsevier). ISBN 1-4160-2984-2
- Canadian Pharmacists Association (2000). Compendium of Pharmaceuticals and Specialties (25th ed.). Toronto, ON: Webcom. ISBN 0-919115-76-4
- Carlson, NR (2001). Physiology of Behavior (7th ed.). Needham Heights, MA: Allyn and Bacon. ISBN 0-205-30840-6
- Gershon, Michael D. (1998). The Second Brain. New York, NY: HarperCollins. ISBN 0-06-018252-0
- Siegal A & Sapru HN (2006) Essential Neuroscience. Revised 1st ed. Philadelphia: Lippincott, Williams & Wilkins. Ch. 15, pp. 255–267.
- Hasselmo, ME. "Neuromodulation and cortical function: Modeling the physiological basis of behavior." Behavioral Brain Research. 1995 Feb; 67(1):1-27. PMID 7748496
- Yu, AJ & Dayan, P. "Uncertainty, neuromodulation, and attention." Neuron. 2005 May 19; 46(4):681-92. PMID 15944135
External links
Cholinergics Receptor ligands Agonists: 77-LH-28-1 • AC-42 • AC-260,584 • Aceclidine • Acetylcholine • AF30 • AF150(S) • AF267B • AFDX-384 • Alvameline • AQRA-741 • Arecoline • Bethanechol • Butyrylcholine • Carbachol • CDD-0034 • CDD-0078 • CDD-0097 • CDD-0098 • CDD-0102 • Cevimeline • cis-Dioxolane • Ethoxysebacylcholine • LY-593,039 • L-689,660 • LY-2,033,298 • McNA343 • Methacholine • Milameline • Muscarine • NGX-267 • Ocvimeline • Oxotremorine • PD-151,832 • Pilocarpine • RS86 • Sabcomeline • SDZ 210-086 • Sebacylcholine • Suberylcholine • Talsaclidine • Tazomeline • Thiopilocarpine • Vedaclidine • VU-0029767 • VU-0090157 • VU-0152099 • VU-0152100 • VU-0238429 • WAY-132,983 • Xanomeline • YM-796
Antagonists: 3-Quinuclidinyl Benzilate • 4-DAMP • Aclidinium Bromide • Anisodamine • Anisodine • Atropine • Atropine Methonitrate • Benactyzine • Benzatropine (Benztropine) • Benzydamine • BIBN 99 • Biperiden • Bornaprine • CAR-226,086 • CAR-301,060 • CAR-302,196 • CAR-302,282 • CAR-302,368 • CAR-302,537 • CAR-302,668 • CS-27349 • Cyclobenzaprine • Cyclopentolate • Darifenacin • DAU-5884 • Dimethindene • Dexetimide • DIBD • Dicyclomine (Dicycloverine) • Ditran • EA-3167 • EA-3443 • EA-3580 • EA-3834 • Elemicin • Etanautine • Etybenzatropine (Ethylbenztropine) • Flavoxate • Himbacine • HL-031,120 • Ipratropium bromide • J-104,129 • Hyoscyamine • Mamba Toxin 3 • Mamba Toxin 7 • Mazaticol • Mebeverine • Methoctramine • Metixene • Myristicin • N-Ethyl-3-Piperidyl Benzilate • N-Methyl-3-Piperidyl Benzilate • Orphenadrine • Otenzepad • Oxybutynin • PBID • PD-102,807 • PD-0298029 • Phenglutarimide • Phenyltoloxamine • Pirenzepine • Piroheptine • Procyclidine • Profenamine • RU-47,213 • SCH-57,790 • SCH-72,788 • SCH-217,443 • Scopolamine (Hyoscine) • Solifenacin • Telenzepine • Tiotropium bromide • Tolterodine • Trihexyphenidyl • Tripitamine • Tropatepine • Tropicamide • WIN-2299 • Xanomeline • Zamifenacin; Others: 1st Generation Antihistamines (Brompheniramine, chlorphenamine, cyproheptadine, dimenhydrinate, diphenhydramine, doxylamine, mepyramine/pyrilamine, phenindamine, pheniramine, tripelennamine, triprolidine, etc) • Tricyclic Antidepressants (Amitriptyline, doxepin, trimipramine, etc) • Tetracyclic Antidepressants (Amoxapine, maprotiline, etc) • Typical Antipsychotics (Chlorpromazine, thioridazine, etc) • Atypical Antipsychotics (Clozapine, olanzapine, quetiapine, etc)Agonists: 5-HIAA • A-84,543 • A-366,833 • A-582,941 • A-867,744 • ABT-202 • ABT-418 • ABT-560 • ABT-894 • Acetylcholine • Altinicline • Anabasine • Anatoxin-a • AR-R17779 • Butyrylcholine • Carbachol • Cotinine • Cytisine • Decamethonium • Desformylflustrabromine • Dianicline • Dimethylphenylpiperazinium • Epibatidine • Epiboxidine • Ethanol • Ethoxysebacylcholine • EVP-4473 • EVP-6124 • Galantamine • GTS-21 • Ispronicline • Lobeline • MEM-63,908 (RG-3487) • Nicotine • NS-1738 • PHA-543,613 • PHA-709,829 • PNU-120,596 • PNU-282,987 • Pozanicline • Rivanicline • Sazetidine A • Sebacylcholine • SIB-1508Y • SIB-1553A • SSR-180,711 • Suberylcholine • TC-1698 • TC-1734 • TC-1827 • TC-2216 • TC-5214 • TC-5619 • TC-6683 • Tebanicline • Tropisetron • UB-165 • Varenicline • WAY-317,538 • XY-4083
Antagonists: 18-Methoxycoronaridine • α-Bungarotoxin • α-Conotoxin • Alcuronium • Amantadine • Anatruxonium • Atracurium • Bupropion (Amfebutamone) • Chandonium • Chlorisondamine • Cisatracurium • Coclaurine • Coronaridine • Dacuronium • Decamethonium • Dextromethorphan • Dextropropoxyphene • Dextrorphan • Diadonium • DHβE • Dimethyltubocurarine (Metocurine) • Dipyrandium • Dizocilpine (MK-801) • Doxacurium • Duador • Esketamine • Fazadinium • Gallamine • Hexafluronium • Hexamethonium (Benzohexonium) • Ibogaine • Isoflurane • Ketamine • Kynurenic acid • Laudexium (Laudolissin) • Levacetylmethadol • Malouetine • Mecamylamine • Memantine • Methadone • Methorphan (Racemethorphan) • Methyllycaconitine • Metocurine • Mivacurium • Morphanol (Racemorphanol) • Neramexane • Nitrous Oxide • Pancuronium • Pempidine • Pentamine • Pentolinium • Phencyclidine • Pipecuronium • Radafaxine • Rapacuronium • Rocuronium • Surugatoxin • Suxamethonium (Succinylcholine) • Thiocolchicoside • Toxiferine • Trimethaphan • Tropeinium • Tubocurarine • Vecuronium • XenonReuptake inhibitors PlasmalemmalCHT InhibitorsHemicholinium-3 (Hemicholine; HC3) • TriethylcholineVAChT InhibitorsEnzyme inhibitors ChAT inhibitors1-(-Benzoylethyl)pyridinium • 2-(α-Naphthoyl)ethyltrimethylammonium • 3-Chloro-4-stillbazole • 4-(1-Naphthylvinyl)pyridine • Acetylseco hemicholinium-3 • Acryloylcholine • AF64A • B115 • BETA • CM-54,903 • CatabolismAChE inhibitorsReversible: Carbamates: Aldicarb • Bendiocarb • Bufencarb • Carbaryl • Carbendazim • Carbetamide • Carbofuran • Chlorbufam • Chloropropham • Ethienocarb • Ethiofencarb • Fenobucarb • Fenoxycarb • Formetanate • Furadan • Ladostigil • Methiocarb • Methomyl • Miotine • Oxamyl • Phenmedipham • Pinmicarb • Pirimicarb • Propamocarb • Propham • Propoxur; Stigmines: Ganstigmine • Neostigmine • Phenserine • Physostigmine • Pyridostigmine • Rivastigmine; Others: Acotiamide • Ambenonium • Donepezil • Edrophonium • Galantamine • Huperzine A • Minaprine • Tacrine • Zanapezil
Irreversible: Organophosphates: Acephate • Azinphos-methyl • Bensulide • Cadusafos • Chlorethoxyfos • Chlorfenvinphos • Chlorpyrifos • Chlorpyrifos-Methyl • Coumaphos • Cyclosarin (GF) • Demeton • Demeton-S-Methyl • Diazinon • Dichlorvos • Dicrotophos • Diisopropyl fluorophosphate (Guthion) • Diisopropylphosphate • Dimethoate • Dioxathion • Disulfoton • EA-3148 • Echothiophate • Ethion • Ethoprop • Fenamiphos • Fenitrothion • Fenthion • Fosthiazate • GV • Isofluorophate • Isoxathion • Malaoxon • Malathion • Methamidophos • Methidathion • Metrifonate • Mevinphos • Monocrotophos • Naled • Novichok agent • Omethoate • Oxydemeton-Methyl • Paraoxon • Parathion • Parathion-Methyl • Phorate • Phosalone • Phosmet • Phostebupirim • Phoxim • Pirimiphos-Methyl • Sarin (GB) • Soman (GD) • Tabun (GA) • Temefos • Terbufos • Tetrachlorvinphos • Tribufos • Trichlorfon • VE • VG • VM • VR • VX; Others: Demecarium • Onchidal (Onchidella binneyi)BChE inhibitorsCymserine * Many of the acetylcholinesterase inhibitors listed above act as butyrylcholinesterase inhibitors.Others Choline (Lecithin) • Citicoline • Cyprodenate • Dimethylethanolamine (DMAE, deanol) • Glycerophosphocholine • Meclofenoxate (Centrophenoxine) • Phosphatidylcholine • Phosphatidylethanolamine • Phosphorylcholine • PirisudanolOthersAcetylcholine releasing agents: α-Latrotoxin • β-Bungarotoxin; Acetylcholine release inhibitors: Botulinum toxin (Botox); Acetylcholinesterase reactivators: Asoxime • Obidoxime • PralidoximeNeurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others See also Template:NeuropeptidesCategories:- Acetylcholine
- Choline esters
- Quaternary ammonium compounds
- Acetate esters
Wikimedia Foundation. 2010.
Look at other dictionaries:
Acetylcholine — Acétylcholine Pour les articles homonymes, voir Ach. Acétylcholine … Wikipédia en Français
ACÉTYLCHOLINE — L’acétylcholine (ACh) est l’ester acétique de la choline, corps basique azoté dérivé de l’ammonium quaternaire. Formule de l’acétylcholine: 廓 acétoxyéthyl triméthyl ammonium. L’ACh a été préparée par voie synthétique en 1867 et peut être obtenue… … Encyclopédie Universelle
acetylcholine — n. 1. a neurotransmitter released by the transmitting dendron at autononmous synapses and at neuromuscular junctions. It is a quaternary amine with an obligatory negative counterion. The nominal formula for the hydroxide form is {C7H17NO3}.… … The Collaborative International Dictionary of English
acetylcholine — acetylcholine. См. ацетилхолин. (Источник: «Англо русский толковый словарь генетических терминов». Арефьев В.А., Лисовенко Л.А., Москва: Изд во ВНИРО, 1995 г.) … Молекулярная биология и генетика. Толковый словарь.
acetylcholine — [as΄i til΄kō′lēn] n. [ ACETYL + CHOLINE] the acetic acid ester of choline, (CH3) 3N(OH)CH2CH2OCOCH3, found in many body tissues, esp. at neuromuscular junctions, acting as a nerve impulse transmitter: its chloride or bromide is used in medicine… … English World dictionary
Acétylcholine — Pour les articles homonymes, voir ACh. Acétylcholine … Wikipédia en Français
acetylcholine — The acetic ester of choline, the neurotransmitter substance at cholinergic synapses, which causes cardiac inhibition, vasodilation, gastrointestinal peristalsis, and other par … Medical dictionary
acetylcholine — n. the acetic acid ester of the organic base choline: the neurotransmitter released at the synapses of parasympathetic nerves and at neuromuscular junctions. After relaying a nerve impulse, acetylcholine is rapidly broken down by the enzyme… … The new mediacal dictionary
acetylcholine — Acetyl ester of choline. Perhaps the best characterized neurotransmitter, particularly at neuromuscular junctions. ACh can be either excitatory or inhibitory, and its receptors are classified as nicotinic or muscarinic, according to their… … Dictionary of molecular biology
acetylcholine — acetilcholinas statusas T sritis chemija formulė Formulę žr. priede. priedas( ai) Grafinis formatas atitikmenys: angl. acetylcholine rus. ацетилхолин ryšiai: sinonimas – 2 etanoiloksietiltrimetilamonis … Chemijos terminų aiškinamasis žodynas
