- Oleamide
-
Oleamide[1] 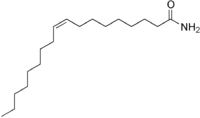 (Z)-Octa-9-decenamideOther namesOleylamide
(Z)-Octa-9-decenamideOther namesOleylamide
9-Octadecenamide
(Z)-9-Octadecenamide
9,10-Octadecenoamide
Oleic acid amide
Cis-9,10-octadecenoamideIdentifiers CAS number 301-02-0 
PubChem 5283387 ChemSpider 4446508 
UNII 7L25QK8BWO 
EC number 206-103-9 ChEBI CHEBI:116314 
ChEMBL CHEMBL15927 
IUPHAR ligand 284 Jmol-3D images Image 1 - O=C(N)CCCCCCC\C=C/CCCCCCCC
Properties Molecular formula C18H35NO Molar mass 281.48 g mol−1 Appearance Creamy solid Density 0.879 g/cm3 Melting point 102-104 °C
Boiling point >200 °C
Solubility in water Insoluble Hazards NFPA 704 Flash point >200 °C  (verify) (what is:
(verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references Oleamide is an amide of the fatty acid oleic acid. It is an endogenous substance: it occurs naturally in the body of animals. It accumulates in the cerebrospinal fluid during sleep deprivation and induces sleep in animals.[2] It is being studied as a potential medical treatment for mood and sleep disorders, and cannabinoid-regulated depression.[3][4]
The mechanism of action of oleamide's sleep inducing effects is an area of current research. It is likely that oleamide interacts with multiple neurotransmitter systems.[5] Oleamide is structurally related to the endogenous cannabinoid anandamide, and has the ability to bind to the CB1 receptor as a full agonist. In addition, oleamide potentiates several serotonin receptors and the GABA(A) receptor, and inhibits gap junction communication.[citation needed]
Synthetically produced oleamide has a variety of industrial uses including as a slip agent, a lubricant, and a corrosion inhibitor.[6]
Oleamide was originally characterized as an endogenous bioactive substance, isolated from the cerebrospinal fluid of sleep deprived cats. It was characterised in 1995 by Benjamin Cravatt III and Richard Lerner at The Scripps Research Institute in La Jolla, CA [7].
Oleamide was found by researchers to be leaking out of polypropylene plastics used in laboratory experiments, affecting experimental results.[8] Since polypropylene is used in a wide number of food containers such as those for yogurt, the problem is being studied.[9]
See also
- Anandamide
- Fatty acid amide hydrolase
- Virodhamine
References
- ^ Oleamide at chemicalland21.com
- ^ Salvador Huitron-Resendiz, Lhys Gombart, Benjamin F. Cravatt, and Steven J. Henriksen (2001). "Effect of Oleamide on Sleep and Its Relationship to Blood Pressure, Body Temperature, and Locomotor Activity in Rats". Experimental Neurology 172 (1): 235–243. doi:10.1006/exnr.2001.7792. PMID 11681856.
- ^ Methods of treating anxiety and mood disorders with oleamide - US Patent 6359010
- ^ Raphael Mechoulam, Ester Fride, Lumír Ondřej Hanuš, Tzviel Sheskin, Tiziana Bisogno, Vincenzo Di Marzo, Michael Bayewitch and Zvi Vogel (1997). "Anandamide may mediate sleep induction". Nature 389 (6646): 25–26. doi:10.1038/37891. PMID 9288961.
- ^ Fedorova I, Hashimoto A, Fecik RA, et al (2001). "Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems". J. Pharmacol. Exp. Ther. 299 (1): 332–42. PMID 11561096.
- ^ Surfactants : Westco Oleamide a Slip Agent In Polyethylene Films[dead link]
- ^ Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA (June 1995). "Chemical characterization of a family of brain lipids that induce sleep". Science 268 (5216): 1506–9. doi:10.1126/science.7770779. PMID 7770779. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=7770779.
- ^ McDonald, RG. et al (2008). "Bioactive Contaminants Leach from Disposable Laboratory Plasticware". Science 322 (5903): 917. doi:10.1126/science.1162395. PMID 18988846.
- ^ Mittelstaedt, Martin (6 November 2008). "Researchers Raise Alarm After Chemical Leak Found In Common Plastic". Globe and Mail. http://www.ewg.org/node/27344. Retrieved 28 December 2009.
Cannabinoids Plant cannabinoids Cannabinoid metabolites 8,11-DiOH-THC · 11-COOH-THC · 11-OH-THC
Endogenous cannabinoids Arachidonoyl ethanolamide (Anandamide or AEA) · 2-Arachidonoylglycerol (2-AG) · 2-Arachidonyl glyceryl ether (noladin ether) · Virodhamine · Palmitoylethanolamide (PEA) · N-Arachidonoyl dopamine (NADA) · Oleamide · RVD-Hpα
Synthetic cannabinoid
receptor agonistsClassical cannabinoids
(Dibenzopyrans)A-40174 · A-41988 · A-42574 · Ajulemic acid · AM-087 · AM-411 · AM-855 · AM-905 · AM-906 · AM-919 · AM-926 · AM-938 · AM-4030 · AMG-1 · AMG-3 · AMG-36 · AMG-41 · Dexanabinol (HU-211) · DMHP · Dronabinol · HHC · HU-210 · JWH-051 · JWH-133 · JWH-139 · JWH-161 · JWH-229 · JWH-359 · KM-233 · L-759,633 · L-759,656 · Levonantradol (CP 50,5561) · Nabazenil · Nabidrox (Canbisol) · Nabilone · Nabitan · Naboctate · O-581 · O-774 · O-806 · O-823 · O-1057 · O-1125 · O-1238 · O-2365 · O-2372 · O-2373 · O-2383 · O-2426 · O-2484 · O-2545 · O-2694 · O-2715 · O-2716 · O-3223 · O-3226 · Parahexyl · Perrottetinene · Pirnabine · THC-O-acetate · THC-O-phosphate
Nonclassical cannabinoidsBenzoylindoles1-Butyl-3-(2-methoxybenzoyl)indole · 1-Butyl-3-(4-methoxybenzoyl)indole · 1-Pentyl-3-(2-methoxybenzoyl)indole · AM-630 · AM-679 · AM-694 · AM-1241 · AM-2233 · GW-405,833 (L-768,242) · Pravadoline · RCS-4 · WIN 54,461
NaphthoylindolesNaphthylmethylindolesJWH-175 · JWH-184 · JWH-185 · JWH-192 · JWH-194 · JWH-195 · JWH-196 · JWH-197 · JWH-199
PhenylacetylindolesCannabipiperidiethanone · JWH-167 · JWH-203 · JWH-249 · JWH-250 · JWH-251 · JWH-302 · RCS-8
NaphthoylpyrrolesEicosanoidsAM-883 · Arachidonyl-2'-chloroethylamide (ACEA) · Arachidonylcyclopropylamide (ACPA) · Methanandamide (AM-356) · O-585 · O-689 · O-1812 · O-1860 · O-1861
Others(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone · N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide · A-796,260 · A-834,735 · A-836,339 · Abnormal cannabidiol · AB-001 · AM-1248 · AZ-11713908 · BAY 38-7271 · BAY 59-3074 · CB-13 · CB-86 · GW-842,166X · JWH-171 · JWH-176 · JTE 7-31 · Leelamine · MDA-19 · O-1918 · O-2220 · Org 28312 · Org 28611 · SER-601 · VSN-16 · WIN 56,098
Allosteric modulators of
cannabinoid receptorsOrg 27569 · Org 27759 · Org 29647
Endocannabinoid
activity enhancersAM-404 · CAY-10401 · CAY-10429 · JZL184 · JZL195 · Arachidonoyl serotonin · O-1624 · PF-04457845 · PF-622 · PF-750 · PF-3845 · PHOP · URB-447 · URB-597 · URB-602 · URB-754 · Genistein · Arvanil · Olvanil · Kaempferol · Biochanin A
Cannabinoid receptor
antagonists and
inverse agonistsAM-251 · AM-281 · AM-630 · BML-190 · CAY-10508 · CB-25 · CB-52 · CB-86 · Drinabant · Hemopressin · Ibipinabant (SLV319) · JTE-907 · LY-320,135 · Taranabant (MK-0364) · MK-9470 · NESS-0327 · O-1184 · O-1248 · O-2050 · O-2654 · Otenabant · Rimonabant (SR141716) · SR144528 · Surinabant (SR147778) · TM-38837 · VCHSR
Neurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Eicosanoids
- Cannabinoids
- Amides
- Hypnotics
- Lipids
Wikimedia Foundation. 2010.

