- (1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone
-
(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone 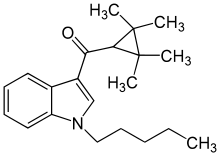
Systematic (IUPAC) name (1-pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone Clinical data Pregnancy cat. ? Legal status uncontrolled Identifiers ATC code ? PubChem CID 44626619 Chemical data Formula C21H29NO Mol. mass 311.461 g/mol SMILES eMolecules & PubChem (1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone is a drug invented by Abbott Laboratories,[1] that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor. It has high affinity for the CB2 receptor with a Ki of 1.8nM but 83x lower affinity for the CB1 receptor with a Ki of 150nM.[2] Chemically it is closely related to other 2,2,3,3-tetramethylcyclopropyl synthetic cannabinoids like A-796,260 and A-834,735 but with a different substitution on the 1-position of indole core, its 1-pentyl group replaced with alkylheterocycles like 1-(2-morpholinoethyl) and 1-(tetrahydropyran-4-ylmethyl).
See also
References
- ^ WO application 2006069196, Pace JM, Tietje K, Dart MJ, Meyer MD, "3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands", published 2006-06-29, assigned to Abbott Laboratories
- ^ Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". J. Med. Chem. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
Further reading
- Poso A, Huffman JW (January 2008). "Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands". Br. J. Pharmacol. 153 (2): 335–46. doi:10.1038/sj.bjp.0707567. PMC 2219524. PMID 17982473. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2219524.
- Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, Zhu CZ, Gauvin D, Pai M, Wetter J, Hsieh GC, Honore P, Frost JM, Dart MJ, Meyer MD, Yao BB, Cox BF, Fox GB (January 2008). "Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI". Br. J. Pharmacol. 153 (2): 367–79. doi:10.1038/sj.bjp.0707506. PMC 2219521. PMID 17965748. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2219521.
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Miller LN, Li L, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (March 2008). "Indol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity". J. Med. Chem. 51 (6): 1904–12. doi:10.1021/jm7011613. PMID 18311894.
Cannabinoids Plant cannabinoids Cannabinoid metabolites 8,11-DiOH-THC · 11-COOH-THC · 11-OH-THC
Endogenous cannabinoids Arachidonoyl ethanolamide (Anandamide or AEA) · 2-Arachidonoylglycerol (2-AG) · 2-Arachidonyl glyceryl ether (noladin ether) · Virodhamine · Palmitoylethanolamide (PEA) · N-Arachidonoyl dopamine (NADA) · Oleamide · RVD-Hpα
Synthetic cannabinoid
receptor agonistsClassical cannabinoids
(Dibenzopyrans)A-40174 · A-41988 · A-42574 · Ajulemic acid · AM-087 · AM-411 · AM-855 · AM-905 · AM-906 · AM-919 · AM-926 · AM-938 · AM-4030 · AMG-1 · AMG-3 · AMG-36 · AMG-41 · Dexanabinol (HU-211) · DMHP · Dronabinol · HHC · HU-210 · JWH-051 · JWH-133 · JWH-139 · JWH-161 · JWH-229 · JWH-359 · KM-233 · L-759,633 · L-759,656 · Levonantradol (CP 50,5561) · Nabazenil · Nabidrox (Canbisol) · Nabilone · Nabitan · Naboctate · O-581 · O-774 · O-806 · O-823 · O-1057 · O-1125 · O-1238 · O-2365 · O-2372 · O-2373 · O-2383 · O-2426 · O-2484 · O-2545 · O-2694 · O-2715 · O-2716 · O-3223 · O-3226 · Parahexyl · Perrottetinene · Pirnabine · THC-O-acetate · THC-O-phosphate
Nonclassical cannabinoidsBenzoylindoles1-Butyl-3-(2-methoxybenzoyl)indole · 1-Butyl-3-(4-methoxybenzoyl)indole · 1-Pentyl-3-(2-methoxybenzoyl)indole · AM-630 · AM-679 · AM-694 · AM-1241 · AM-2233 · GW-405,833 (L-768,242) · Pravadoline · RCS-4 · WIN 54,461
NaphthoylindolesNaphthylmethylindolesJWH-175 · JWH-184 · JWH-185 · JWH-192 · JWH-194 · JWH-195 · JWH-196 · JWH-197 · JWH-199
PhenylacetylindolesCannabipiperidiethanone · JWH-167 · JWH-203 · JWH-249 · JWH-250 · JWH-251 · JWH-302 · RCS-8
NaphthoylpyrrolesEicosanoidsAM-883 · Arachidonyl-2'-chloroethylamide (ACEA) · Arachidonylcyclopropylamide (ACPA) · Methanandamide (AM-356) · O-585 · O-689 · O-1812 · O-1860 · O-1861
Others(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone · N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide · A-796,260 · A-834,735 · A-836,339 · Abnormal cannabidiol · AB-001 · AM-1248 · AZ-11713908 · BAY 38-7271 · BAY 59-3074 · CB-13 · CB-86 · GW-842,166X · JWH-171 · JWH-176 · JTE 7-31 · Leelamine · MDA-19 · O-1918 · O-2220 · Org 28312 · Org 28611 · SER-601 · VSN-16 · WIN 56,098
Allosteric modulators of
cannabinoid receptorsOrg 27569 · Org 27759 · Org 29647
Endocannabinoid
activity enhancersAM-404 · CAY-10401 · CAY-10429 · JZL184 · JZL195 · N-arachidonoyl-serotonin · O-1624 · PF-04457845 · PF-622 · PF-750 · PF-3845 · PHOP · URB-447 · URB-597 · URB-602 · URB-754 · Genistein · Arvanil · Olvanil · Kaempferol · Biochanin A
Cannabinoid receptor
antagonists and
inverse agonistsAM-251 · AM-281 · AM-630 · BML-190 · CAY-10508 · CB-25 · CB-52 · CB-86 · Drinabant · Hemopressin · Ibipinabant (SLV319) · JTE-907 · LY-320,135 · Taranabant (MK-0364) · MK-9470 · NESS-0327 · O-1184 · O-1248 · O-2050 · O-2654 · Otenabant · Rimonabant (SR141716) · SR144528 · Surinabant (SR147778) · TM-38837 · VCHSR
This cannabinoid related article is a stub. You can help Wikipedia by expanding it.
