- JWH-018
-
JWH-018 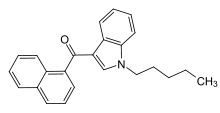
Systematic (IUPAC) name Naphthalen-1-yl-(1-pentylindol-3-yl)methanone Clinical data Pregnancy cat. ? Legal status Schedule I (US) Routes Smoked, Oral Identifiers CAS number 209414-07-3 
ATC code ? PubChem CID 10382701 ChemSpider 8558143 
ChEMBL CHEMBL561013 
Chemical data Formula C24H23NO Mol. mass 341.45 g/mol Physical data Solubility in water hydrophobic, n/a mg/mL (20 °C)  (what is this?) (verify)
(what is this?) (verify)JWH-018 (1-pentyl-3-(1-naphthoyl)indole) or AM-678[1] is an analgesic chemical from the naphthoylindole family, which acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2.[2][3][4][5] It produces effects in animals similar to those of THC, a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis.
Contents
History
John W. Huffman, an organic chemist at Clemson University, synthesized analogues and metabolites of Δ9-tetrahydrocannabinol (THC), the principal active component of cannabis. JWH-018 is one of these analogues, with studies showing an affinity for the cannabinoid (CB1) receptor five times greater than that of THC. Cannabinoid receptors are found in mammalian brain and spleen tissue; however, the structural details of the active sites are currently unknown.[6][7]
On December 15, 2008, it was reported by the German pharmaceutical company THC Pharm that JWH-018 was found as one of the active components in at least three versions of the herbal blend Spice, which has been sold as an incense in a number of countries around the world since 2002.[8][9][10] An analysis of samples acquired four weeks after the German prohibition of JWH-018 took place found that the compound had been replaced with JWH-073.[11]
Pharmacology
JWH-018 is a full agonist of both the CB1 and CB2 cannabinoid receptors, with a reported binding affinity of 9.00±5.00 nM at CB1 and 2.94±2.65 nM at CB2.[12]
JWH-018 administered to rats resulted in the excretion of an indole-N-desalkyl metabolite as well as several hydroxylated metabolites in urine. The highest signals were observed for the hydroxylated N-desalkyl metabolites. Hydroxylation took place on the side chain and in both aromatic systems, the naphthalene and the indole rings, as could be shown by mass shift of the corresponding fragments and by MS3 experiments.[13] Human metabolites were similar although most metabolism took place on the indole ring and pentyl side chain, and the hydroxylated metabolites were extensively conjugated with glucuronide.[14]
Usage
At least one case of JWH-018 dependence has been reported. On October 15, 2011, coroner reports released to the media reveal that the death of a South Carolina college basketball player was attributed to "drug toxicity and organ failure" caused by JWH-018. The user consumed JWH-018 daily for eight months. Withdrawal symptoms were similar to those experienced as a result of cannabis dependence.[15] JWH-018 has been shown to cause profound changes in CB1 receptor density following administration, causing desensitization to its effects more rapidly than related cannabinoids.[5]
Compared to THC, which is a partial agonist at CB1 receptors, JWH-018 (and many of its analogues) are full agonists. This means that users may experience far more intense effects compared to smoking cannabis. THC has been shown to inhibit GABA neurotransmission in the brain via several pathways.[16][17] JWH-018 may cause intense anxiety, agitation, and, in rare cases (generally with non-regular JWH users), has been assumed to have been the cause of seizures and convulsions by inhibiting GABA neurotransmission more effectively than THC. Cannabinoid receptor full agonists may present serious dangers to the user when used to excess.[18]
Various physical and psychological adverse effects have been reported from JWH-018 use. One study reported psychotic relapses and anxiety symptoms in well-treated patients with mental illness following JWH-018 inhalation.[19] Due to concerns about the potential of JWH-018 and other synthetic cannabinoids to cause psychosis in vulnerable individuals, it has been recommended that people with risk factors for psychotic illnesses (like a past or family history of psychosis) not use these substances.[20]
According to the writer Kevin Keck, who sampled various brands of herbal incense containing JWH-018 for research on the subject, "A small amount does produce visual distortions and a marijuana-like buzz. I can easily understand how individuals with little or no experience in navigating this psychic terrain could experience extreme panic attacks that prompt them to seek medical attention...It is not a problem that responsible people use [JWH-018], it is a serious problem that naïve and foolish people use [it]. It is the same problem that surrounds alcohol and any other number of legal prescription drugs."[21]
Detection in biological fluids
JWH-018 usage is not detectable with the usual immunoassay screening methods employed for detecting cannabis use from urine specimens. Determination of the parent drug in serum or its metabolites in urine has been accomplished by GC-MS or LC-MS. Serum JWH-018 concentrations are generally in the 1-10 μg/L range during the first few hours after recreational usage. The major urinary metabolite is a compound that is monohydroxylated on the omega minus one carbon atom of the alkyl side chain. A lesser metabolite monohydroxylated on the omega (terminal) position was present in the urine of 6 users of the drug at concentrations of 6-50 μg/L, primarily as a glucuronide conjugate.[22][23][24][25][26][27][28][29][30]
Legal status
Country Date of Ban Notes Austria The Austrian Ministry of Health announced on 18 December 2008 that Spice would be controlled under Paragraph 78 of their drug law on the grounds that it contains an active substance which affects the functions of the body, and the legality of JWH-018 is under review. Belarus 1 January 2010 Canada June 3, 2010 JWH-018 is not a controlled substance in Canada. Note: the most current CDSA can be found here[31] France February 24, 2009 [32][33] Germany 22 January 2009 [34] Ireland 11 May 2010 An immediate ban was announced on 11 May 2010 by Minister for Health Mary Harney.[35] Italy 2 July 2010 [36] Latvia 28 November 2009 Poland [32] South Korea 1 July 2009 [37] Sweden 30 July 2009 A bill to ban JWH-018 was accepted on 30 July 2009 and was in effect on 15 September 2009.[38] Estonia 24 July 2009 Romania 15 February 2010 Russia 22 January 2010 Ukraine 31 May 2010 United Kingdom 23 December 2009 [39] United States 1 March 2011 JWH-018 and four similar cannabinoids were classified as Schedule I controlled substances by the Drug Enforcement Administration on March 1, 2011, making their possession and use illegal in all 50 states.[40] The law allows for a one-year DEA study of the effects of JWH-018 on the human body to determine and whether it should be permanently classified as Schedule I in the United States.[41] See also
References
- ^ "Department of Justice :: Drug Enforcement Administration". 2011-03-01. http://www.scribd.com/doc/49741625/DEA-Rule-on-Synthetic-Cannabinoids. Retrieved 2011-03-02.
- ^ Aung, M. M.; et al. (2000). "Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding". Drug and Alcohol Dependence 60 (2): 133–140. doi:10.1016/S0376-8716(99)00152-0. PMID 10940540.
- ^ US patent 6900236, Alexandros Makriyannis, Hongfeng Deng, "Cannabimimetic indole derivatives", issued 2005-05-31
- ^ US patent 7241799, Alexandros Makriyannis, Hongfeng Deng, "Cannabimimetic indole derivatives", issued 2007-07-10
- ^ a b B.K. Atwood et. al., "JWH018, a common constituent of 'Spice' herbal blends, is a potent and efficacious cannabinoid CB1 receptor agonist." British Journal of Pharmacology, Vol. 160, No. 3. 585-593. 2010.[1]
- ^ "Clemson University :: Department of Chemistry". Clemson.edu. http://www.clemson.edu/chemistry/people/huffman.html. Retrieved 2010-08-23.
- ^ 11:10. "Drugs Forum". Drugs Forum. http://www.drugs-forum.com/forum/showthread.php?s=118de94c4da38df748848101980625d4&t=71910. Retrieved 2010-08-23.
- ^ Gefährlicher Kick mit Spice (German)
- ^ Erstmals Bestandteile der Modedroge „Spice“ nachgewiesen (German)
- ^ Spice enthält chemischen Wirkstoff (German)
- ^ Lindigkeit, Rainer; Boehme, A; Eiserloh, I; Luebbecke, M; Wiggermann, M; Ernst, L; Beuerle, T (30 October 2009). "Spice: A never ending story?". Forensic science international (Forensic Science International) 191 (1): 58–63. doi:10.1016/j.forsciint.2009.06.008. PMID 19589652
- ^ Aung, M.M., Griffin, G., Huffman, J.W., et al. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug and Alcohol Dependence 60 133-140 (2000)
- ^ Studies on the metabolism of JWH-018 and of a homologue of CP 47,497, pharmacologically active ingredients of different misused incense (“Spice”) using GC-MS and LC-MSn techniques - T. Kraemer, K.Y. Rust, M.R. Meyer, D.K. Wissenbach, D. Bregel, M. Hopf, H.H. Maurer, J. Wilske (Institute of Legal Medicine, Saarland University, 66421 Homburg, Germany http://www.gtfch.org/cms/images/stories/media/tk/tk76_2/abstractsvortraege.pdf
- ^ Sobolevsky T, Prasolov I, Rodchenkov G (July 2010). "Detection of JWH-018 metabolites in smoking mixture post-administration urine". Forensic Science International 200 (1-3): 141–7. doi:10.1016/j.forsciint.2010.04.003. PMID 20430547.
- ^ Zimmermann, US; Winkelmann, PR; Pilhatsch, M; Nees, JA; Spanagel, R; Schulz, K (2009). "Withdrawal Phenomena and Dependence Syndrome After the Consumption of "Spice Gold"". Dtsch Arztebl Int 106 (27): 464–467. doi:10.3238/arztebl.2009.0464. PMC 2719097. PMID 19652769. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2719097.
- ^ Laaris, Nora; Good, Cameron H.; Lupica, Carl R. (July – August 2010). "Δ9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus". Neuropharmacology 59 (1–2): 121–127. doi:10.1016/j.neuropharm.2010.04.013. PMC 2882293. PMID 20417220. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2882293. Retrieved 2011-07-26.
- ^ Hoffman, Alexander F.; Lupica, Carl R. (2000-04-01). "Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus.". The Journal of Neuroscience 20 (7): 2470–2479. ISSN 0270-6474. PMID 10729327. http://www.jneurosci.org/content/20/7/2470.long. Retrieved 2011-07-26.
- ^ European Monitoring Centre for Drugs and Drug Addiction. "Understanding the Spice Phenomenon." 2009. ISBN 978-92-9168-411-3.[2]
- ^ Every-Palmer, S. Synthetic cannabinoid use and psychosis: an explorative study. Journal of Drug and Alcohol Dependence 2011. [Epub ahead of print].
- ^ Every-Palmer, S. (2010), WARNING: LEGAL SYNTHETIC CANNABINOID-RECEPTOR AGONISTS SUCH AS JWH-018 MAY PRECIPITATE PSYCHOSIS IN VULNERABLE INDIVIDUALS. Addiction, 105: 1859–1860. doi: 10.1111/j.1360-0443.2010.03119.x.
- ^ . Creative Loafing. August 24, 2010. http://charlotte.creativeloafing.com/gyrobase/a_user_s_guide_to_legal_weed_/Content?oid=1028428 User's Guide to "Legal Weed"
- ^ Möller I, Wintermeyer A, Bender K, et al. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test. Anal. Sep 24, 2010. [Epub ahead of print]
- ^ Teske J, Weller JP, Fieguth A, et al. Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography-tandem mass spectrometry. J Chrom. B 878: 2659-2663, 2010.
- ^ Auwärter, V.; Dresen, S.; Weinmann, W.; Müller, M.; Pütz, M.; Ferreirós, N. (2009). "'Spice' and other herbal blends: harmless incense or cannabinoid designer drugs?". Journal of mass spectrometry : JMS 44 (5): 832–837. doi:10.1002/jms.1558. PMID 19189348. Free version
- ^ Zimmermann, U.; Winkelmann, P.; Pilhatsch, M.; Nees, J.; Spanagel, R.; Schulz, K. (2009). "Withdrawal phenomena and dependence syndrome after the consumption of "spice gold"". Deutsches Arzteblatt international 106 (27): 464–467. doi:10.3238/arztebl.2009.0464. PMC 2719097. PMID 19652769. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2719097.
- ^ Sobolevsky, T.; Prasolov, I.; Rodchenkov, G. (2010). "Detection of JWH-018 metabolites in smoking mixture post-administration urine". Forensic science international 200 (1–3): 141–147. doi:10.1016/j.forsciint.2010.04.003. PMID 20430547.
- ^ Beuck S, Möller I, Thomas A, et al. Structure characterisation of urinary metabolites of the cannabimimetic JWH-018 using chemically synthesised reference material for the support of LC-MS/MS-based drug testing. Anal. Bioanal. Chem. 2011 Apr 1 [Epub ahead of print] PMID:21455647
- ^ Moran CL, Le VH, Chimalakonda KC, et al. Quantitative Measurement of JWH-018 and JWH-073 Metabolites Excreted in Human Urine. Anal. Chem. 2011 Apr 20 [Epub ahead of print] PMID:21506519
- ^ Logan BK, Kacinko SL, McMullin MM, et al. Identification of primary JWH-018 and JWH-073 metabolites in human urine. NMS Labs Technical Bulletin, May 25, 2011. http://toxwiki.wikispaces.com/file/view/JWH_metabolites_Technical_Bulletin_Final_v1.1.pdf
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, CA, 2011, p. 1566. http://www.biomedicalpublications.com/spice.pdf
- ^ "Controlled Drugs and Substances Act". Laws.justice.gc.ca. 2010-08-16. http://laws.justice.gc.ca/en/C-38.8/. Retrieved 2010-08-23.
- ^ a b "EMCDDA | Drug profile: Synthetic cannabinoids and 'Spice'". Emcdda.europa.eu. 2010-08-17. http://www.emcdda.europa.eu/publications/drug-profiles/synthetic-cannabinoids#control. Retrieved 2010-08-23.
- ^ http://www.afssaps.fr/var/afssaps_site/storage/original/application/d23d05edc58479d91c803b496017f073.pdf
- ^ BGBl I Nr. 3 vom 21.01.2009, 22. BtMÄndV vom 19. Januar 2009, S. 49–50.
- ^ Many head shop products banned - Irish Times.
- ^ http://www.politicheantidroga.it/comunicazione/comunicati/2010/luglio/spice,-n-joy-e-mefedrone-da-oggi-stupefacenti.aspx (Italian)
- ^ 최연희 (2 July 2009). "1일부터 ‘5-메오-밉트’ 등 향정신성의약품 지정". 헬스코리아뉴스. http://www.hkn24.com/news/articleView.html?idxno=28611. Retrieved 18 February 2010.
- ^ http://www.regeringen.se/sb/d/12102/a/130038 (Swedish)
- ^ Ford, Richard (2009-12-23). "Three legal highs banned after deaths linked to the drugs". The Times (London). http://business.timesonline.co.uk/tol/business/law/article6965663.ece. Retrieved 2010-05-07.
- ^ Cook, Morgan (2011-02-28). "Synthetic marijuana illegal as of Tuesday". North County Times (San Diego). http://www.nctimes.com/news/local/sdcounty/article_d000d0ec-653e-51a8-bc3d-55e144f415c1.html. Retrieved 2011-02-28.
- ^ Meserve, Jeanne (2011-02-28). "DEA imposes "emergency" ban to control synthetic marijuana". CNN. http://www.cnn.com/2011/US/02/28/dea.synthetic.marijuana/index.html?iref=allsearch. Retrieved 2011-03-01.
http://www.nzherald.co.nz/nz/news/article.cfm?c_id=1&objectid=10716926
External links
- JWH-018 Report Psychonaut Web Mapping Research Project
Cannabinoids Plant cannabinoids Cannabinoid metabolites 8,11-DiOH-THC · 11-COOH-THC · 11-OH-THC
Endogenous cannabinoids Arachidonoyl ethanolamide (Anandamide or AEA) · 2-Arachidonoylglycerol (2-AG) · 2-Arachidonyl glyceryl ether (noladin ether) · Virodhamine · Palmitoylethanolamide (PEA) · N-Arachidonoyl dopamine (NADA) · Oleamide · RVD-Hpα
Synthetic cannabinoid
receptor agonistsClassical cannabinoids
(Dibenzopyrans)A-40174 · A-41988 · A-42574 · Ajulemic acid · AM-087 · AM-411 · AM-855 · AM-905 · AM-906 · AM-919 · AM-926 · AM-938 · AM-4030 · AMG-1 · AMG-3 · AMG-36 · AMG-41 · Dexanabinol (HU-211) · DMHP · Dronabinol · HHC · HU-210 · JWH-051 · JWH-133 · JWH-139 · JWH-161 · JWH-229 · JWH-359 · KM-233 · L-759,633 · L-759,656 · Levonantradol (CP 50,5561) · Nabazenil · Nabidrox (Canbisol) · Nabilone · Nabitan · Naboctate · O-581 · O-774 · O-806 · O-823 · O-1057 · O-1125 · O-1238 · O-2365 · O-2372 · O-2373 · O-2383 · O-2426 · O-2484 · O-2545 · O-2694 · O-2715 · O-2716 · O-3223 · O-3226 · Parahexyl · Perrottetinene · Pirnabine · THC-O-acetate · THC-O-phosphate
Nonclassical cannabinoidsBenzoylindoles1-Butyl-3-(2-methoxybenzoyl)indole · 1-Butyl-3-(4-methoxybenzoyl)indole · 1-Pentyl-3-(2-methoxybenzoyl)indole · AM-630 · AM-679 · AM-694 · AM-1241 · AM-2233 · GW-405,833 (L-768,242) · Pravadoline · RCS-4 · WIN 54,461
NaphthoylindolesAM-1220 · AM-1221 · AM-1235 · AM-2201 · AM-2232 · JWH-007 · JWH-015 · JWH-018 · JWH-019 · JWH-073 · JWH-081 · JWH-098 · JWH-116 · JWH-122 · JWH-149 · JWH-164 · JWH-182 · JWH-193 · JWH-198 · JWH-200 · JWH-210 · JWH-398 · JWH-424 · WIN 55,212-2
NaphthylmethylindolesJWH-175 · JWH-184 · JWH-185 · JWH-192 · JWH-194 · JWH-195 · JWH-196 · JWH-197 · JWH-199
PhenylacetylindolesCannabipiperidiethanone · JWH-167 · JWH-203 · JWH-249 · JWH-250 · JWH-251 · JWH-302 · RCS-8
NaphthoylpyrrolesEicosanoidsAM-883 · Arachidonyl-2'-chloroethylamide (ACEA) · Arachidonylcyclopropylamide (ACPA) · Methanandamide (AM-356) · O-585 · O-689 · O-1812 · O-1860 · O-1861
OthersA-796,260 · A-834,735 · A-836,339 · Abnormal cannabidiol · AB-001 · AM-1248 · AZ-11713908 · BAY 38-7271 · BAY 59-3074 · CB-13 · CB-86 · GW-842,166X · JWH-171 · JWH-176 · JTE 7-31 · Leelamine · MDA-19 · O-1918 · O-2220 · Org 28312 · Org 28611 · SER-601 · UR-12 · VSN-16 · WIN 56,098
Allosteric modulators of
cannabinoid receptorsOrg 27569 · Org 27759 · Org 29647
Endocannabinoid
activity enhancersAM-404 · CAY-10401 · CAY-10429 · JZL184 · JZL195 · N-arachidonoyl-serotonin · O-1624 · PF-04457845 · PF-622 · PF-750 · PF-3845 · PHOP · URB-447 · URB-597 · URB-602 · URB-754 · Genistein · Arvanil · Olvanil · Kaempferol · Biochanin A
Cannabinoid receptor
antagonists and
inverse agonistsAM-251 · AM-281 · AM-630 · BML-190 · CAY-10508 · CB-25 · CB-52 · CB-86 · Drinabant · Hemopressin · Ibipinabant (SLV319) · JTE-907 · LY-320,135 · Taranabant (MK-0364) · MK-9470 · NESS-0327 · O-1184 · O-1248 · O-2050 · O-2654 · Otenabant · Rimonabant (SR141716) · SR144528 · Surinabant (SR147778) · TM-38837 · VCHSR
Categories:- Naphthoylindoles
- JWH cannabinoids
- AM cannabinoids
- Euphoriants
- Synthetic Cannabis
Wikimedia Foundation. 2010.

