- N-Arachidonoyl dopamine
-
N-Arachidonoyl dopamine 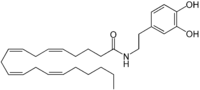 (5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)-ethyl]icosa-5,8,11,14-tetraenamideOther namesNADA
(5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)-ethyl]icosa-5,8,11,14-tetraenamideOther namesNADAIdentifiers CAS number 199875-69-9 
PubChem 5282105 Jmol-3D images Image 1 - CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCC1=CC(=C(C=C1)O)O
Properties Molecular formula C28H41NO3 Molar mass 439.63 g/mol  dopamine (verify) (what is:
dopamine (verify) (what is:  /
/ ?)
?)
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa)Infobox references N-Arachidonoyl dopamine (NADA) is an endocannabinoid which acts as an agonist of the CB1 receptor[1] and the transient receptor potential V1 (TRPV1) ion channel. Its discovery was described in 2002 by an academic research group from Italy and the USA. It was found in the brain of rats, with especially high concentrations in the hippocampus, cerebellum and striatum. It activates the TRPV1 channel with an EC50 of approximately of 50nM. The high potency makes it the putative endogenous TRPV1 agonist.[2]
See also
- Endocannabinoid
References
- ^ Ralevic V. (July 2003). "Cannabinoid modulation of peripheral autonomic and sensory neurotransmission.". European journal of pharmacology 472 (1-2): 1–21. doi:10.1016/S0014-2999(03)01813-2. PMID 12860468.
- ^ Huang SM, Bisogno T, Trevisani M, et al. (June 2002). "An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors". Proceedings of the National Academy of Sciences of the United States of America 99 (12): 8400–5. doi:10.1073/pnas.122196999. PMC 123079. PMID 12060783. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=123079.
External links
- General information about NADA.
Cannabinoids Plant cannabinoids Cannabinoid metabolites 8,11-DiOH-THC · 11-COOH-THC · 11-OH-THC
Endogenous cannabinoids Arachidonoyl ethanolamide (Anandamide or AEA) · 2-Arachidonoylglycerol (2-AG) · 2-Arachidonyl glyceryl ether (noladin ether) · Virodhamine · Palmitoylethanolamide (PEA) · N-Arachidonoyl dopamine (NADA) · Oleamide · RVD-Hpα
Synthetic cannabinoid
receptor agonistsClassical cannabinoids
(Dibenzopyrans)A-40174 · A-41988 · A-42574 · Ajulemic acid · AM-087 · AM-411 · AM-855 · AM-905 · AM-906 · AM-919 · AM-926 · AM-938 · AM-4030 · AMG-1 · AMG-3 · AMG-36 · AMG-41 · Dexanabinol (HU-211) · DMHP · Dronabinol · HHC · HU-210 · JWH-051 · JWH-133 · JWH-139 · JWH-161 · JWH-229 · JWH-359 · KM-233 · L-759,633 · L-759,656 · Levonantradol (CP 50,5561) · Nabazenil · Nabidrox (Canbisol) · Nabilone · Nabitan · Naboctate · O-581 · O-774 · O-806 · O-823 · O-1057 · O-1125 · O-1238 · O-2365 · O-2372 · O-2373 · O-2383 · O-2426 · O-2484 · O-2545 · O-2694 · O-2715 · O-2716 · O-3223 · O-3226 · Parahexyl · Perrottetinene · Pirnabine · THC-O-acetate · THC-O-phosphate
Nonclassical cannabinoidsBenzoylindoles1-Butyl-3-(2-methoxybenzoyl)indole · 1-Butyl-3-(4-methoxybenzoyl)indole · 1-Pentyl-3-(2-methoxybenzoyl)indole · AM-630 · AM-679 · AM-694 · AM-1241 · AM-2233 · GW-405,833 (L-768,242) · Pravadoline · RCS-4 · WIN 54,461
NaphthoylindolesNaphthylmethylindolesJWH-175 · JWH-184 · JWH-185 · JWH-192 · JWH-194 · JWH-195 · JWH-196 · JWH-197 · JWH-199
PhenylacetylindolesCannabipiperidiethanone · JWH-167 · JWH-203 · JWH-249 · JWH-250 · JWH-251 · JWH-302 · RCS-8
NaphthoylpyrrolesEicosanoidsAM-883 · Arachidonyl-2'-chloroethylamide (ACEA) · Arachidonylcyclopropylamide (ACPA) · Methanandamide (AM-356) · O-585 · O-689 · O-1812 · O-1860 · O-1861
Others(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone · N-(S)-Fenchyl-1-(2-morpholinoethyl)-7-methoxyindole-3-carboxamide · A-796,260 · A-834,735 · A-836,339 · Abnormal cannabidiol · AB-001 · AM-1248 · AZ-11713908 · BAY 38-7271 · BAY 59-3074 · CB-13 · CB-86 · GW-842,166X · JWH-171 · JWH-176 · JTE 7-31 · Leelamine · MDA-19 · O-1918 · O-2220 · Org 28312 · Org 28611 · SER-601 · VSN-16 · WIN 56,098
Allosteric modulators of
cannabinoid receptorsOrg 27569 · Org 27759 · Org 29647
Endocannabinoid
activity enhancersAM-404 · CAY-10401 · CAY-10429 · JZL184 · JZL195 · Arachidonoyl serotonin · O-1624 · PF-04457845 · PF-622 · PF-750 · PF-3845 · PHOP · URB-447 · URB-597 · URB-602 · URB-754 · Genistein · Arvanil · Olvanil · Kaempferol · Biochanin A
Cannabinoid receptor
antagonists and
inverse agonistsAM-251 · AM-281 · AM-630 · BML-190 · CAY-10508 · CB-25 · CB-52 · CB-86 · Drinabant · Hemopressin · Ibipinabant (SLV319) · JTE-907 · LY-320,135 · Taranabant (MK-0364) · MK-9470 · NESS-0327 · O-1184 · O-1248 · O-2050 · O-2654 · Otenabant · Rimonabant (SR141716) · SR144528 · Surinabant (SR147778) · TM-38837 · VCHSR
Neurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Cannabinoids
- Vanilloids
- Eicosanoids
- Catechols
- Fatty acid amides
Wikimedia Foundation. 2010.
