- Octopamine
-
Octopamine 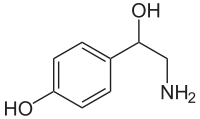
Systematic (IUPAC) name (RS)-4-(2-amino-1-hydroxy-ethyl)phenol Clinical data Pregnancy cat. ? Legal status ℞ Prescription only Routes Oral Pharmacokinetic data Half-life 15 Minutes in insects. Theorized to be longer in vertebrates. Identifiers CAS number 104-14-3 
ATC code C01CA18 PubChem CID 4581 ChemSpider 4420 
UNII 14O50WS8JD 
ChEBI CHEBI:17134 
ChEMBL CHEMBL53929 
Synonyms Norsympathol, Norsynephrine, para-Octopamine, beta-Hydroxytyramine, para-hydroxy-phenyl-ethanolamine Chemical data Formula C8H11NO2 Mol. mass 153.178 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Octopamine (β,4-dihydroxyphenethylamine) is an endogenous biogenic amine that is closely related to norepinephrine, and has effects on the adrenergic and dopaminergic systems. It is also found naturally in numerous plants, including bitter orange.[1][2] Biosynthesis of the D-(—)-enantiomer of octopamine is by β-hydroxylation of tyramine via the enzyme dopamine β-hydroxylase. Under the trade names Epirenor, Norden, and Norfen, octopamine is also used clinically as a sympathomimetic agent.[3][4]
Contents
Role in invertebrates
Octopamine was first discovered by Italian scientist Vittorio Erspamer in 1948[5] in the salivary glands of the octopus and has since been found to act as neurotransmitter, neurohormone and neuromodulator in invertebrates. It is widely used in energy-demanding behaviors by all insects, crustaceans (crabs, lobsters, crayfish), and spiders. Such behaviors include flying, egg-laying, and jumping.
The best-understood role for octopamine is in the locust jump. Here it modulates muscle activity, making the leg muscles contract more effectively. This is at least in part due to an increase in the rate of contraction and of relaxation.
In the honey bee and fruit fly, octopamine has a major role in learning and memory. In the firefly, octopamine release leads to light production in the lantern.
Octopamine also plays a role in mollusks, though the role of octopamine has been examined only in the central nervous system of the model organism, the pond snail.
Heberlein et al. [6] have conducted studies of alcohol tolerance in fruit flies; they found that a mutation that caused octopamine deficiency also caused lower alcohol tolerance.[7][8][9][10]
The emerald cockroach wasp stings the host for its larvae (a cockroach) in the head ganglion (brain). The venom blocks octopamine receptors[11] and the cockroach fails to show normal escape responses, grooming itself excessively. It becomes docile and the wasp leads it to the wasp's den by pulling its antenna like a leash. [12]
Role in vertebrates
In vertebrates, octopamine replaces norepinephrine in sympathetic neurons with chronic use of monoamine oxidase inhibitors. It may be responsible for the common side effect of orthostatic hypotension with these agents, though there is also evidence that it is actually mediated by increased levels of N-acetylserotonin.
In mammals, octopamine may mobilize the release of fat from adipocytes (fat cells), which has led to its promotion on the internet as a slimming aid. However, the released fat is likely to be promptly taken up into other cells, and there is no evidence that octopamine facilitates weight loss. Octopamine may also increase blood pressure significantly when combined with other stimulants, as in some weight loss supplements. [13] [14]
Owing to lack of research, much is not known about octopamine or its role in humans.
See also
References
- ^ Tang, F; Tao, L; Luo, X; Ding, L; Guo, M; Nie, L; Yao, S (2006). "Determination of octopamine, synephrine and tyramine in Citrus herbs by ionic liquid improved 'green' chromatography". Journal of chromatography. A 1125 (2): 182–8. doi:10.1016/j.chroma.2006.05.049. PMID 16781718.
- ^ Jagiełło-Wójtowicz E (1979). "Mechanism of central action of octopamine". Pol J Pharmacol Pharm 31 (5): 509–16. PMID 121158.
- ^ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0. http://books.google.com/?id=5GpcTQD_L2oC&lpg=PA1499&dq=octopamine%20index&pg=PA756#v=onepage&q.
- ^ "Pharmacognosy And Pharmacobiotechnology - Google Books". http://books.google.com/books?id=PlMi4XvHCYoC&lpg=PA810&dq=Epirenor%20octopamine&pg=PA810#v=onepage&q&f=false.
- ^ Erspamer, V., Active substances in the posterior salivary glands of Octopoda. 2. Tyramine and octopamine (oxyoctopamine) (1948). "Active Substances in the Posterior Salivary Glands of Octopoda. II. Tyramine and Octopamine (Oxyoctopamine)". Acta Pharmacologica et Toxicologica 4 (3–4): 224–247. doi:10.1111/j.1600-0773.1948.tb03345.x.
- ^ Heberlein, U.; Wolf, FW; Rothenfluh, A; Guarnieri, DJ (2004). "Molecular Genetic Analysis of Ethanol Intoxication in Drosophila melanogaster". Integrative and Comparative Biology 44 (4): 269–74. doi:10.1093/icb/44.4.269. PMID 21676709.
- ^ Moore, M. S., Dezazzo, J., Luk, A. Y., Tully, T., Singh, C. M., and Heberlein, U. (1998). "Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP pathway". Cell 93 (6): 997–1007. doi:10.1016/S0092-8674(00)81205-2. PMID 9635429.
- ^ Tecott, L. H. and Heberlein, U. (1998). "Y do we drink?". Cell 95 (6): 733–735. doi:10.1016/S0092-8674(00)81695-5. PMID 9865690.
- ^ Bar Flies: What our insect relatives can teach us about alcohol tolerance., Ruth Williams, Naked Scientist
- ^ ‘Hangover gene’ is key to alcohol tolerance, Gaia Vince, NewScientist.com news service, 22 August 2005
- ^ How to make a zombie cockroach, Nature News, 29 September 2007
- ^ Gal, Ram; Rosenberg, Lior Ann; Libersat, Frederic (22 November 2005). "Parasitoid wasp uses a venom cocktail injected into the brain to manipulate the behavior and metabolism of its cockroach prey". Archives of Insect Biochemistry and Physiology 60 (4): 198–208. doi:10.1002/arch.20092. PMID 16304619. http://www3.interscience.wiley.com/journal/112152224/abstract.
- ^ Minerd, Jeff (12 September 2005). "Ephedra-Free Supplements Not Necessarily Risk-Free". MedPage Today. http://www.medpagetoday.com/Cardiology/Hypertension/tb1/1713?pfc=101&spc=235. Retrieved 2009-09-12.
- ^ Haller, C; Benowitz, N; Jacobiii, P (2005). "Hemodynamic effects of ephedra-free weight-loss supplements in humans". The American Journal of Medicine 118 (9): 998–1003. doi:10.1016/j.amjmed.2005.02.034. PMID 16164886.
Further reading
- P.D. Evans, "Octopamine", in Comprehensive Insect Physiology, 11, 499, Oxford University Press 1985.
Cardiac stimulants excluding cardiac glycosides (C01C) Adrenergic and
dopaminergic agentsαβmixedBothUnknown/ungroupedPhosphodiesterase inhibitors (PDE3I) Other cardiac stimulants Adrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tedatioxetine • Teniloxazine • Tramadol • ZiprasidoneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsPhenethylamines Phenethylamines Psychedelics: 2C-B • 2C-B-FLY • 2C-C • 2C-D • 2C-E • 2C-F • 2C-G • 2C-I • 2C-N • 2C-P • 2C-SE • 2C-T • 2C-T-2 • 2C-T-4 • 2C-T-7 • 2C-T-8 • 2C-T-9 • 2C-T-13 • 2C-T-15 • 2C-T-17 • 2C-T-21 • 2C-TFM • 2C-YN • Allylescaline • DESOXY • Escaline • Isoproscaline • Jimscaline • Macromerine • MEPEA • Mescaline • Metaescaline • Methallylescaline • Proscaline • Psi-2C-T-4 • TCB-2
Stimulants: 2-OH-PEA • β-Me-PEA • Hordenine • N-Me-PEA • Phenethylamine (PEA)
Entactogens: Lophophine • MDPEA • MDMPEA
Others: BOH • DMPEAAmphetamines
PhenylisopropylaminesPsychedelics: 3C-BZ • 3C-E • 3C-P • Aleph • Beatrice • Bromo-DragonFLY • D-Deprenyl • DMA • DMCPA • DMMDA • DOB • DOC • DOEF • DOET • DOI • DOM • DON • DOPR • DOTFM • Ganesha • MMDA • MMDA-2 • Psi-DOM • TMA • TeMA
Stimulants: 4-MA • 4-MMA • 4-MTA • 5-IT • Alfetamine • Amfecloral • Amfepentorex • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • Benfluorex • Benzphetamine • Cathine • Clobenzorex • Dimethylamphetamine • Ephedrine (EPH) • Ethylamphetamine • Fencamfamine • Fencamine • Fenethylline • Fenfluramine (Dexfenfluramine) • Fenproporex • Fludorex • Furfenorex • Isopropylamphetamine • Lefetamine • Mefenorex • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methoxyphenamine • MMA • Norfenfluramine • Oxilofrine • Ortetamine • PBA • PCA • Phenpromethamine • PFA • PFMA • PIA • PMA • PMEA • PMMA • Phenylpropanolamine (PPA) • Prenylamine • Propylamphetamine • Pseudoephedrine (PSE) • Sibutramine • Tiflorex (Flutiorex) • Tranylcypromine • Xylopropamine • Zylofuramine
Entactogens: 5-APDB • 6-APB • 6-APDB • EDA • IAP • MDA • MDEA • MDHMA (FLEA) • MDMA ("Ecstasy") • MDOH • MMDMA • NAP • TAP
Others: Amiflamine • DFMDA • D-Deprenyl • L-Deprenyl (Selegiline)Phentermines Stimulants: Chlorphentermine • Cloforex • Clortermine • Etolorex • Mephentermine • Pentorex (Phenpentermine) • Phentermine
Entactogens: MDPH • MDMPHCathinones Stimulants: Amfepramone • Brephedrone • Buphedrone • Bupropion (Amfebutamone) • Cathinone (Propion) • Dimethylcathinone (Dimethylpropion, Metamfepramone) • Ethcathinone (Ethylpropion) • Flephedrone • Methcathinone (Methylpropion) • Mephedrone • Methedrone
Entactogens: Ethylone • MethylonePhenylisobutylamines Phenylalkylpyrrolidines Stimulants: α-PBP • α-PPP • α-PVP • MDPBP • MDPPP • MDPV • MOPPP • MPBP • MPHP • MPPP • Naphyrone • PEP • Prolintane • PyrovaleroneCatecholamines
(and relatives..)6-FNE • 6-OHDA • α-Me-DA • α-Me-TRA • Adrenochrome • Ciladopa • D-DOPA (Dextrodopa) • Dopamine • Epinephrine (Adrenaline) • Epinine • Fenclonine • Ibopamine • L-DOPA (Levodopa) • L-DOPS (Droxidopa) • L-Phenylalanine • L-Tyrosine • meta-Octopamine • meta-Tyramine • Metanephrine • Metirosine • Methyldopa • Nordefrin (Levonordefrin) • Norepinephrine (Noradrenaline) • Normetanephrine • para-Octopamine • para-TyramineMiscellaneous Amidephrine • Arbutamine • Cafedrine • Denopamine • Dobutamine • Dopexamine • Etafedrine • Ethylnorepinephrine • Etilefrine • Famprofazone • Gepefrine • Isoprenaline (Isoproterenol) • Isoetarine • Metaraminol • Metaterol • Methoxamine • Norfenefrine • Orciprenaline • Phenylephrine (Neosynephrine) • Phenoxybenzamine • Prenalterol • Pronethalol • Propranolol • Salbutamol (Albuterol; Levosalbutamol) • Synephrine (Oxedrine) • Theodrenaline • XamoterolNeurotransmitters Amino acids Alanine · Aspartate · Cycloserine · DMG · GABA · Glutamate · Glycine · Hypotaurine · Kynurenic acid (Transtorine) · NAAG (Spaglumic acid) · NMG (Sarcosine) · Serine · Taurine · TMG (Betaine)
Endocannabinoids 2-AG · 2-AGE (Noladin ether) · AEA (Anandamide) · NADA · OAE (Virodhamine) · Oleamide · PEA (Palmitoylethanolamide) · RVD-Hpα · Hp (Hemopressin)
Gasotransmitters Monoamines Purines Trace amines 3-ITA · 5-MeO-DMT · Bufotenin · DMT · NMT · Octopamine · Phenethylamine · Synephrine · Thyronamine · Tryptamine · Tyramine
Others 1,4-BD · Acetylcholine · GBL · GHB · Histamine
See also Template:NeuropeptidesCategories:- Phenethylamines
- Alpha-adrenergic agonists
- Phenols
Wikimedia Foundation. 2010.
