- Prazosin
-
Prazosin 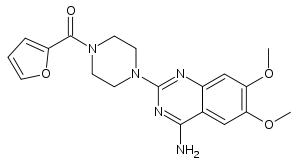
Systematic (IUPAC) name 2-[4-(2-furoyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine Clinical data Trade names Minipress AHFS/Drugs.com monograph MedlinePlus a682245 Pregnancy cat. ? Legal status POM (UK) Routes Oral Pharmacokinetic data Bioavailability ~60% Protein binding 97% Half-life 2–3 hours Identifiers CAS number 19216-56-9 
ATC code C02CA01 PubChem CID 4893 IUPHAR ligand 503 DrugBank APRD00020 ChemSpider 4724 
UNII XM03YJ541D 
KEGG D08411 
ChEBI CHEBI:8364 
ChEMBL CHEMBL2 
Chemical data Formula C19H21N5O4 Mol. mass 383.401 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Prazosin, trade names Minipress, Vasoflex, Pressin and Hypovase, is a sympatholytic drug used to treat high blood pressure and Anxiety, PTSD and Panic Disorder. It belongs to the class of alpha-adrenergic blockers. Specifically, prazosin is selective for the alpha-1 receptors on vascular smooth muscle. These receptors are responsible for the vasoconstrictive action of norepinephrine, which would normally raise blood pressure and cause increase in anxiety and panic. By blocking these receptors, prazosin reduces blood pressure and reduces anxiety and panic.
Contents
Use
Prazosin is orally active and has a minimal effect on cardiac function due to its alpha-1 receptor selectivity. However, when prazosin is initially started, heart rate and contractility go up in order to maintain the pre-treatment blood pressures because the body has reached homeostasis at its abnormally high blood pressure. The blood pressure lowering effect becomes apparent when prazosin is taken for longer periods of time. The heart rate and contractility go back down over time and blood pressure decreases.
The antihypertensive characteristics of prazosin make it a second-line choice for the treatment of high blood pressure.[1]
Prazosin is also useful in treating urinary hesitancy associated with prostatic hyperplasia, blocking alpha-1 receptors, which control constriction of both the prostate and ureters. Although not a first line choice for either hypertension or prostatic hyperplasia, it is a choice for patients who present with both problems concomitantly.[1]
This medication has shown to be effective in treating severe nightmares in children, associated with PTSD symptoms.[2] Veterans have also been treated successfully at the Oregon VA for sleep disturbance related to PTSD. Doses are lower for this purpose than for control of blood pressure.[2]
Prazosin holds promise as a pharmacologic treatment for alcohol dependence after a 2009 pilot trial l was completed. A larger controlled Phase II trial "Clinical Trial of the Adrenergic Alpha-1 Antagonist Prazosin for Alcohol Dependence" is currently underway, set to be completed November 2013.
Side effects
Side effects of prazosin include orthostatic hypotension, syncope, and nasal congestion. The orthostatic hypotension and syncope are associated with the body's poor ability to control blood pressure without active alpha-adrenergic receptors. Patients on prazosin should be told not to stand up too quickly, since their poor baroreflex may cause them to faint as all their blood rushes to their feet. The nasal congestion is due to dilation of vessels in the nasal mucosa.
One phenomenon associated with prazosin is known as the "first dose response", in which the side effects of the drug, especially orthostatic hypotension and fainting, are especially pronounced in the first dose.
One very rare side effect of prazosin (and doxazosin) is priapism.[3][4]
Another possible side effect is dreaming while awake or hallucinations of wakefulness while falling asleep on the medication (see oneirophrenia) .
Prazosin in management of PTSD
Prazosin has been reported to be useful in management of anxiety, such as PTSD. Raskin and colleagues studied the efficacy of prazosin for PTSD among ten Vietnam combat veterans in a 20-week double-blind crossover protocol with a two-week drug washout to allow for return to baseline. The CAPS and the Clinical Global Impressions-Change scale (CGI-C) were the primary outcome measures. Patients who were taking prazosin had a robust improvement in overall sleep quality (effect size, 1.6) and recurrent distressing dreams (effect size, 1.9). In each of the PTSD symptom clusters the effect size was medium to large: .7 for reexperiencing or intrusion, .6 for avoidance and numbing, and .9 for hyperarousal. The reduction in CGI-C scores (overall PTSD severity and function at endpoint) also reflected a large effect size (1.4). Prazosin appears to have promise as an effective treatment for PTSD-related sleep disturbance, including trauma-related nightmares, as well as overall Anxiety and PTSD symptoms.[5]
Prazosin in scorpion stings
Since 1983 prazosin has revolutionized the management of severe scorpion stings.[6]
Chemistry
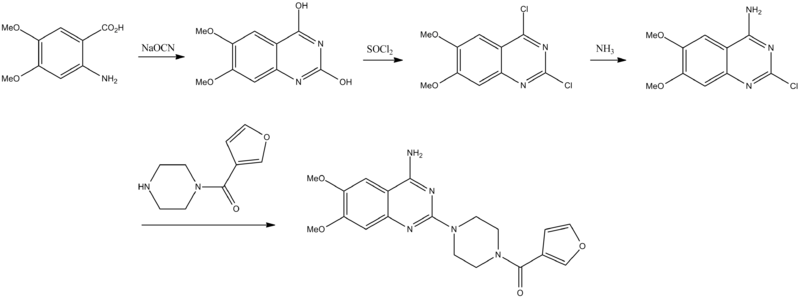
Prazosin, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)-piperazine, is synthesized from 2-amino-4,5-dimethoxybenzoic acid, which upon reaction with sodium cyanate undergoes heterocyclation into 2,4-dihydroxy-6,7-dimethoxyquinazoline. Substituting hydroxyl groups of this compound with chlorine atoms by reaction with thionyl chloride, or a mixture of phosphorus oxychloride with phosphorus pentachloride gives 2,4-dichloro-6,7-dimethoxyquinazoline. Upon subsequent reaction with ammonia, the chlorine atom at C4 of the pyrimidine ring is replaced with an amino group, which leads to the formation of 4-amino-2-chloro-6,7-dimethoxyquinazoline. Introducing this into a reaction with 1-(2-furoyl)piperazine gives prazosin.
- H.-J.E. Hess, U.S. Patent 3,511,836 (1970).
- H.-J.E. Hess, U.S. Patent 3,635,979 (1972).
- H.-J.E. Hess, U.S. Patent 3,663,706 (1972).
- Pfizer & Co., Inc., GB 1156973 (1970).
- E. Honkanen, A. Pippuri, P. Kairisalo, M. Koivisto, S. Tuorni, J. Heterocycl. Chem., 17, 797(1980).
- H.-J.E. Hess, U.S. Patent 3,935,213 (1976).
- Ph.D. Hammen, U.S. Patent 4,062,844 (1977).
- R.R. Crenshaw, G.M. Luke, R.A. Portike, U.S. Patent 4,138,561 (1979).
- A.K. Pippuri, E.J. Honkman, BE 861821 (1977).
- A.K. Pippuri, E.J. Honkman, BE 861822 (1977).
References
- ^ a b Shen, Howard (2008). Illustrated Pharmacology Memory Cards: PharMnemonics. Minireview. p. 13. ISBN 1-59541-101-1.
- ^ a b "Drug Helps PTSD Nightmares" (Press release). Department of Veteran Affairs. March 30, 2008. http://www.research.va.gov/news/press_releases/ptsd-033007.cfm. Retrieved 2009-03-16.
- ^ Bhalla AK, Hoffbrand BI, Phatak PS, Reuben SR (October 1979). "Prazosin and priapism". Br Med J 2 (6197): 1039. doi:10.1136/bmj.2.6197.1039. PMC 1596841. PMID 519276. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1596841.
- ^ Avisrror MU, Fernandez IA, Sánchez AS, García-Pando AC, Arias LM, del Pozo JG (January 2000). "Doxazosin and priapism". J. Urol. 163 (1): 238. doi:10.1016/S0022-5347(05)68018-4. PMID 10604360. http://linkinghub.elsevier.com/retrieve/pii/S0022-5347(05)68018-4.
- ^ Raskind MA, Peskind ER, Kanter ED, et al: Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo controlled study. American Journal of Psychiatry 160:371–373, 2003
- ^ Bawaskar HS, Bawaskar PH (January 2007). "Utility of scorpion antivenin vs prazosin in the management of severe Mesobuthus tamulus (Indian red scorpion) envenoming at rural setting". J Assoc Physicians India 55: 14–21. PMID 17444339.
Raskind MA, Peskind ER, Kanter ED, et al: Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo controlled study. American Journal of Psychiatry 160:371–373, 2003
Sympatholytic (and closely related) antihypertensives (C02) Sympatholytics
(antagonize α-adrenergic
vasoconstriction)CentralAdrenergic release inhibitorsBethanidine • Bretylium • Debrisoquine • Guanadrel • Guanazodine • Guanethidine • Guanoclor • Guanoxan • Guanazodine • Guanoxabenz • GuanoxanImidazoline receptor agonistPeripheralIndirectTyrosine hydroxylase inhibitorDirectNon-selective α blockerOther antagonists #WHO-EM. ‡Withdrawn from market. Clinical trials: †Phase III. §Never to phase III Adrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Teniloxazine • Tramadol • ZiprasidoneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugs Categories:- Alpha blockers
- Furans
- Quinazolines
- Antihypertensive agents
- Piperazines
- Amides
- Phenol ethers
Wikimedia Foundation. 2010.
