- Metoprolol
-
Metoprolol 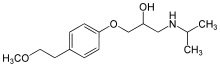
Systematic (IUPAC) name (RS)-1-(Isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol Clinical data AHFS/Drugs.com monograph MedlinePlus a682864 Licence data US FDA:link Pregnancy cat. C(AU) C(US) Legal status ℞ Prescription only Routes Oral, IV Pharmacokinetic data Bioavailability 12% Metabolism Hepatic via CYP2D6, CYP3A4 Half-life 3-7 hours Excretion Renal Identifiers CAS number 51384-51-1 
ATC code C07AB02 PubChem CID 4171 IUPHAR ligand 553 DrugBank APRD00208 ChemSpider 4027 
UNII GEB06NHM23 
KEGG D02358 
ChEBI CHEBI:6904 
ChEMBL CHEMBL13 
Chemical data Formula C15H25NO3 Mol. mass 267.364 g/mol SMILES eMolecules & PubChem Physical data Melt. point 120 °C (248 °F)  (what is this?) (verify)
(what is this?) (verify)Metoprolol (
 /mɛˈtoʊproʊlɑːl/) is a selective β1 receptor blocker used in treatment of several diseases of the cardiovascular system, especially hypertension. The active substance metoprolol is employed either as metoprolol succinate or metoprolol tartrate (where 100 mg metoprolol tartrate corresponds to 95 mg metoprolol succinate). The tartrate is an immediate-release and the succinate is an extended-release formulation[1] .
/mɛˈtoʊproʊlɑːl/) is a selective β1 receptor blocker used in treatment of several diseases of the cardiovascular system, especially hypertension. The active substance metoprolol is employed either as metoprolol succinate or metoprolol tartrate (where 100 mg metoprolol tartrate corresponds to 95 mg metoprolol succinate). The tartrate is an immediate-release and the succinate is an extended-release formulation[1] .Contents
Medical uses
Metoprolol is used for a number of conditions including: hypertension, angina, acute myocardial infarction, supraventricular tachycardia, ventricular tachycardia, congestive heart failure, and prevention of migraine headaches.[2]
- Treatment of heart failure.[3]
- Vasovagal syncope[4]
- Adjunct in treatment of hyperthyroidism
- Long QT syndrome, especially for patients with asthma, as metoprolol's β1 selectivity tends to interfere less with asthma drugs which are often β2-adrenergic receptor-agonist drugs[citation needed]
Due to its selectivity in blocking the beta1 receptors in the heart, metoprolol is also prescribed for off-label use in performance anxiety, social anxiety disorder, and other anxiety disorders.
Metabolism
Metoprolol undergoes a-hydroxylation and O-demethylation as a substrate of the cytochrome liver enzymes CYP2D6 [5] and a small percentage by CYP3A4.
Adverse effects
Side effects, especially with higher dosages, include the following: dizziness, drowsiness, fatigue, diarrhea, unusual dreams, ataxia, trouble sleeping, depression, and vision problems. It may also reduce blood flow to the hands and feet, causing them to feel numb and cold; smoking may worsen this effect.[6]
Serious side effects which are advised to be reported immediately include, but are not limited to, symptoms of bradycardia (a very slow heartbeat (less than 50 bpm)), persistent symptoms of dizziness, fainting and unusual fatigue, bluish discoloration of the fingers and toes, numbness/tingling/swelling of the hands or feet, sexual dysfunction, erectile dysfunction (impotence), hair loss, mental/mood changes, depression, trouble breathing, cough, dyslipidemia, and increased thirst. Other highly unlikely symptoms include easy bruising or bleeding, persistent sore throat or fever, yellowing skin or eyes, stomach pain, dark urine, and persistent nausea. Symptoms of an allergic reaction include: rash, itching, swelling, and severe dizziness. Taking it with alcohol might cause mild body rashes and therefore is not recommended.[6]
Precautions
Metoprolol may worsen the symptoms of heart failure in some patients, who may experience chest pain or discomfort; dilated neck veins; extreme fatigue; irregular breathing; an irregular heartbeat; shortness of breath; swelling of the face, fingers, feet, or lower legs; weight gain; or wheezing.[7]
This medicine may cause changes in blood sugar levels or cover up signs of low blood sugar, such as a rapid pulse rate.[7]
This medicine may cause some people to become less alert than they are normally, making it dangerous for them to drive, use machines, or do other things.[7]
Overdosage
Excessive doses of metoprolol can cause severe hypotension, bradycardia, metabolic acidosis, seizures and cardiorespiratory arrest. Blood or plasma concentrations may be measured to confirm a diagnosis of poisoning in hospitalized patients or to assist in a medicolegal death investigation. Plasma levels are usually less than 200 μg/L during therapeutic administration, but can range from 1-20 mg/L in overdose victims.[8][9][10]
Physical properties
Metoprolol has a very low melting point, around 45 °C (113 °F). Because of this metoprolol is always manufactured in a salt-based solution, as drugs with melting points below 100 °C are difficult to work with in a manufacturing environment. The free base exists as a waxy white solid, and the tartrate salt is finer crystalline material.(Metox-Wockhardt)
Pharmacology
- Selective
- Moderately lipophilic
- Without intrinsic sympathomimetic activity (ISA)
- With weak membrane stabilizing activity
- Short half-life, therefore must be taken at least twice daily or as a slow-release preparation
- Decreases heart rate, contractility and cardiac output, therefore decreasing blood pressure
- Metabolized in the liver to inactive metabolite
Chemistry
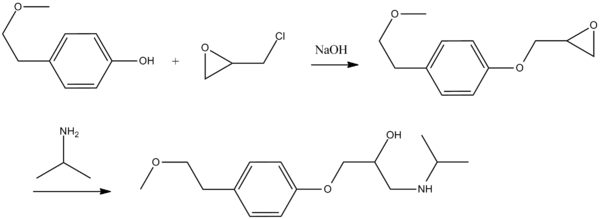
Metoprolol, 1-(iso-propylamino)-3-[4′(2-methoxyethyl)phenoxy]-2-propanol, is synthesized by reacting 4-(2-methoxyethyl)phenol with epichlorohydrin in the presence of a base, isolating 1,2-epoxy-3-[4′(2-methoxyethyl)phenoxy]propane, the subsequent reaction with isopropylamine, gives an opening of the epoxide ring and leads to the formation of metoprolol.
- P.A.E. Carlsson, S.A.I. Carlsson, H.R. Corrodi, L.Ek, B.A.H. Ablad, A.E. Brandstrom, U.S. Patent 3,873,600 (1975).
- A.E. Brandstrom, P.A.E. Carlsson, S.A.I. Carlsson, H.R. Corrodi, L.Ek, DE 2106209
(1971).
Brand names
It is marketed under the brand name Lopressor by Novartis, and Toprol-XL (in the USA); Selokeen (in the Netherlands); as Minax by Alphapharm (in Australia), Metrol by Arrow Pharmaceuticals (in Australia), as Betaloc by AstraZeneca, as Bloxan by Krka (company) (in Slovenia), as Neobloc by Unipharm (in Israel), Presolol by Hemofarm (in Serbia) and as Corvitol by Berlin-Chemie AG (in Germany). In India, this drug is available under the brand names of Metxl, Metolar and Starpress. A number of generic products are available as well.
References
- ^ Pharmacist's Letter/Prescriber's Letter 25 (250302). 2009. http://www.ncbop.org/PDF/MetoprololShortageMarch2009.pdf. Retrieved 3 June 2011.
- ^ "Metoprolol". The American Society of Health-System Pharmacists. http://www.drugs.com/monograph/metoprolol-succinate.html. Retrieved 3 April 2011.
- ^ "Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF)". Lancet 353 (9169): 2001–7. June 12 1999. doi:10.1016/S0140-6736(99)04440-2. PMID 10376614.
- ^ Zhang Q, Jin H, Wang L, Chen J, Tang C, Du J (April 2008). "Randomized comparison of metoprolol versus conventional treatment in preventing recurrence of vasovagal syncope in children and adolescents". Medical science monitor : international medical journal of experimental and clinical research 14 (4): CR199–203. PMID 18376348. http://www.medscimonit.com/fulltxt.php?ICID=850297.
- ^ Clin Pharmacokinet. 2005;44(10):1067-81. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Swaisland HC, Ranson M, Smith RP, Leadbetter J, Laight A, McKillop D, Wild MJ.
- ^ a b Metoprolol, drugs.com
- ^ a b c MayoClinic.com Drug Information Metoprolol Precautions
- ^ Page C, Hacket LP, Isbister GK. The use of high-dose insulin-glucose euglycemia in beta-blocker overdose: a case report. J. Med. Tox. 5: 139-143, 2009.
- ^ Albers S, Elshoff JP, Völker C, Richter A, Läer S. HPLC quantification of metoprolol with solid-phase extraction for the drug monitoring of pediatric patients. Biomed. Chromatogr. 19: 202-207, 2005.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1023-1025.
External links
- AstraZeneca's page for Toprol-XL
- Novartis's page for Lopressor (PDF)
- U.S. National Library of Medicine: Drug Information Portal - Metoprolol
Beta blockers (C07) Beta, nonselective Alprenolol • Bopindolol • Bupranolol • Carteolol • Cloranolol • Mepindolol • Nadolol • Oxprenolol • Penbutolol • Pindolol • Propranolol • Sotalol • Tertatolol • TimololBeta1 selective Acebutolol • Atenolol • Betaxolol • Bevantolol • Bisoprolol • Celiprolol • Epanolol • Esmolol • Nebivolol • Metoprolol • Practolol • S-atenolol • TalinololAlpha + beta Other/ungrouped Categories:- Beta blockers
- Ethers
- Phenol ethers
- Alcohols
Wikimedia Foundation. 2010.