- Trazodone
-
Trazodone 

Systematic (IUPAC) name 2-{3-[4-(3-chlorophenyl)piperazin-1-yl]propyl}[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one Clinical data AHFS/Drugs.com monograph MedlinePlus a681038 Pregnancy cat. C(US) Legal status Unscheduled;
Rx onlyRoutes Oral Pharmacokinetic data Bioavailability High Metabolism Hepatic Half-life 3-6 hours Excretion 20% feces,
80% urineIdentifiers CAS number 19794-93-5 
ATC code N06AX05 PubChem CID 5533 IUPHAR ligand 213 DrugBank APRD00533 ChemSpider 5332 
UNII YBK48BXK30 
KEGG D08626 
ChEBI CHEBI:9654 
ChEMBL CHEMBL621 
Chemical data Formula C19H22ClN5O Mol. mass 371.864 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Trazodone (also sold under the brand names Desyrel, Oleptro, Beneficat, Deprax, Desirel, Molipaxin, Thombran, Trazorel, Trialodine, Trittico, and Mesyrel) is an antidepressant of the serotonin antagonist and reuptake inhibitor (SARI) class. It is a phenylpiperazine compound. Trazodone also has anxiolytic, and hypnotic effects.[1] Trazodone has considerably less prominent anticholinergic (dry mouth, constipation, tachycardia) and sexual side effects than most of the tricyclic antidepressants (TCAs).
Contents
History
Trazodone was originally discovered and developed in Italy in the 1960s by Angelini Research Laboratories as a second-generation antidepressant. It was developed according to the mental pain hypothesis, which was postulated from studying patients and which proposes that major depression is associated with a decreased pain threshold.[2] Trazodone was patented and marketed in many countries all over the world. It was approved by the Food and Drug Administration (FDA) at the end of 1981.
Indications
- Unipolar depression, with or without anxiety
- Bipolar depression, in some circumstances
- Insomnia[3][4][5] (in some countries, this is an off-label use)
- Control of nightmares or other sleep disturbances[6]
Off-label and investigational uses
- Fibromyalgia[7]
- Panic disorder[8]
- Diabetic neuropathy[9]
- Bulimia nervosa[10]
- Obsessive-compulsive disorder (OCD)[11][12]
- Alcohol withdrawal[13][14][15]
- Schizophrenia and other psychosis[16][17]
- Complex Regional Pain Syndrome[citation needed]
Pharmacology
Binding profile
Trazodone behaves as an antagonist at all of the following receptors except 5-HT1A where it acts as a partial agonist similarly to buspirone and tandospirone but with greater intrinsic activity in comparison:[18][19][20][21][22][23]
- 5-HT1A receptor (Kd = 78 nM)
- 5-HT2A receptor (Ki = 13 nM)
- 5-HT2C receptor (Ki = 192 nM)
- α1-adrenergic receptor (Kd = 39 nM)
- α2-adrenergic receptor (Kd = 405 nM)
- H1 receptor (Kd = 725 nM)
It is an inhibitor of the following transporters as well:[24]
- SERT (Kd = 160 nM)
The affinities listed are the means of selected values from the references included.
Clinical effects
Trazodone acts predominantly as a 5-HT2A receptor antagonist to mediate its therapeutic benefits against anxiety and depression.[25] Trazodone's inhibitory effects on serotonin reuptake and 5-HT2C receptors are relatively weak (~15-fold lower than for 5-HT2A) and contribute only lightly to its overall effects.[25] Hence, trazodone does not have similar properties to selective serotonin reuptake inhibitors (SSRIs)[25] and is not particularly associated with increased appetite and weight gain, unlike other 5-HT2C antagonists like mirtazapine.[26][27] Moderate 5-HT1A partial agonism (6-fold lower than 5-HT2A) is likely to contribute to trazodone's antidepressant and anxiolytic actions to some extent as well.[22][23][28]
Trazodone's potent α1-adrenergic blockade (~3-fold lower relative to 5-HT2A) may cause some side effects like orthostatic hypotension and sedation.[29] Conversely, along with 5-HT2A antagonism, it may underlie trazodone's efficacy as a hypnotic. This seems possible as trazodone's antihistamine activity is relatively weak and probably clinically insignificant; hence, it cannot explain trazodone's sleep-inducing/enhancing effects. Notably, trazodone lacks any affinity for muscarinic acetylcholine receptors and therefore does not produce anticholinergic side effects.
mCPP, a non-selective serotonin receptor agonist and serotonin releasing agent is a common active metabolite of trazodone, nefazodone, etoperidone, and mepiprazole, and it has been suggested that it may play a role in trazodone's therapeutic benefits.[30][31][32] However, scientific research has not supported this hypothesis, and mCPP may actually antagonize trazodone's efficacy as well as produce additional side effects.[33][34][35][36][37]
Pharmacokinetics
Trazodone is well absorbed after oral administration with mean peak blood levels obtained at approximately 1 hour after ingestion. Absorption is somewhat delayed and enhanced by food. The mean blood elimination half-life is biphasic: the first phase's half-life is 3–6 hours, and the following phase's half-life is 5–9 hours. The drug is extensively metabolized with 3 or 4 major metabolites having been identified in the human body, particularly mCPP,[38] which may contribute to the side effect profile of trazodone. mCPP has been shown to activate numerous serotonin receptors, including 5-HT2C. Due to the short half-life of trazodone, if a dose is taken at night, mCPP would be present in the body during the following day, causing symptoms such as anorexia (behavioral symptoms), anxiety, hypolocomotion, headache, and depression. Approximately 70–75% of 14C-labelled trazodone was found to be excreted in the urine within 72 hours.[39] Trazodone is highly protein-bound.
As a consequence of the production of mCPP as a metabolite, patients administered Trazodone may test positive on Ecstasy EMIT II urine tests for the presence of MDMA.[40]
Warnings
- If the patient has a known hypersensitivity to trazodone
- If the patient is under eighteen years of age and combines with other antidepressant medications it may increase the possibility of suicidal thoughts or actions.[41]
Precautions
Trazodone is metabolised by CYP3A4, a liver enzyme.[38] Inhibition of this enzyme by various other substances may delay its degradation, leading to high blood levels of trazodone. CYP3A4 may be inhibited by many other medications, herbs, and foods, and as such, trazodone may interact with these substances. One drug-food interaction is grapefruit juice. Drinking grapefruit juice is discouraged in patients taking trazodone. One glass of grapefruit juice occasionally is not likely to have this effect on most people, but drinking large amounts, or drinking it regularly is proven to affect trazodone's clearance.
The possibility of suicide in depressed patients remains during treatment and until significant remission occurs. The number of tablets prescribed at any one time should take into account this possibility, and patients with suicidal ideation should never have access to large quantities of trazodone.
Trazodone has been reported to cause seizures in a small number of patients who took it concurrently with other anti seizure medications.[42]
While trazodone is not a true member of the SSRI class of antidepressants, it does still share many properties of the SSRIs, especially the possibility of discontinuation syndrome if the medication is stopped too quickly.[43] Care must therefore be taken when coming off the medication, usually by a gradual process of tapering down the dose over a period of time.
A person who abruptly stops taking trazodone, even in doses as low as 25 mg (common for use as a sleep aid for people with anxiety disorders), may experience adverse mental reactions such as emotional instability, depressed mood, and suicidal thoughts. Although such warnings may be included in printed materials supplied with the drug, physicians prescribing trazodone, particularly those who are not psychiatrists, might not give oral warnings.
Pregnancy and lactation
- Pregnancy: Sufficient data in humans is lacking. Use should be justified by the severity of the condition to be treated.
- Lactation: Sufficient data in humans is also lacking. Additionally, trazodone may be found in the maternal milk in significant concentrations. Women should not breastfeed while taking trazodone.
Side effects
Adverse reactions reported include the following:[44]
- Headache or heaviness in head
- Nausea, vomiting, or bad taste in mouth
- Dry mouth
- Stomach pain
- Diarrhea
- Constipation
- Changes in appetite or weight
- Weakness or tiredness
- Nervousness
- Decreased ability to concentrate or remember things
- Confusion
- Nightmares
- Muscle pain
- Sweating
- Blurred vision
- Tired, red, or itchy eyes
- Ringing in ears
Serious side effects
"Some side effects can be serious. If you experience any of the following symptoms or those listed in the IMPORTANT WARNING section, call your doctor immediately:"[44]
"IMPORTANT WARNING: ... You should know that your mental health may change in unexpected ways when you take trazodone or other antidepressants even if you are an adult over age 24. You may become suicidal, especially at the beginning of your treatment and any time that your dose is increased or decreased. You, your family, or your caregiver should call your doctor right away if you experience any of the following symptoms: new or worsening depression; thinking about harming or killing yourself, or planning or trying to do so; extreme worry; agitation; panic attacks; difficulty falling asleep or staying asleep; aggressive behavior; irritability; acting without thinking; severe restlessness; and frenzied abnormal excitement."
- Chest pain
- Fast, pounding, or irregular heartbeat
- Shortness of breath
- Fever, sore throat, chills, or other signs of infection
- Hives
- Skin rash
- Itching
- Difficulty breathing or swallowing
- Swelling of the face, throat, tongue, lips, eyes, hands, feet, ankles, or lower legs
- Hoarseness
- Decreased coordination
- Uncontrollable shaking of a part of the body
- Numbness, burning, or tingling in the arms, legs, hands, or feet
- Dizziness or lightheadedness
- Fainting
- Painful erection that lasts longer than normal
Cardiac arrhythmia
Recent clinical studies in patients with pre-existing cardiac disease indicate that trazodone may be arrhythmogenic in some patients in that population. Arrhythmia identified include isolated PVC's, ventricular couplets, and in two patients short episodes (three to four beats) of ventricular tachycardia. There have also been several post-marketing reports of arrhythmia in trazodone-treated patients who have pre-existing cardiac disease and in some patients who did not have pre-existing cardiac disease. Until the results of prospective studies are available, patients with pre-existing cardiac disease should be closely monitored, particularly for cardiac arrhythmias. Trazodone is not recommended for use during the initial recovery phase of myocardial infarction.
Priapism
Trazodone has been associated with the occurrence of priapism, likely due to its antagonism at α-adrenergic receptors.[45] Priapism is a potentially harmful medical condition in which the erect penis does not return to its flaccid state (despite the absence of both physical and psychological stimulation) within four hours. In approximately 33% of the cases reported, surgical intervention was required and, in a portion of these cases, permanent impairment of erectile function or impotence resulted.[44]
In women, a similar condition of persistent arousal can be caused and is called persistent genital arousal disorder.[46]
Other
Rare cases of idiosyncratic hepatotoxicity have been observed, possibly due to the formation of reactive metabolites.[47]
Elevated prolactin concentrations have been observed in patients taking trazodone.[48]
It has been concluded that Trazodone impairs motor healing in brain-injured rats.[49]
Occupational hazards
Since trazodone may impair the mental and/or physical abilities required for performance of potentially hazardous tasks, such as operating an automobile or machinery, the patient should be cautioned not to engage in such activities while impaired.
Laboratory tests
It is recommended that white blood cell and differential counts should be performed in patients who develop sore throat, fever, or other signs of infection or blood dyscrasia and trazodone should be discontinued if the white blood cell or absolute neutrophil count falls below normal.[citation needed]
Drug interactions
Trazodone may enhance the effects of alcohol, barbiturates, and other CNS depressants; patients should be cautioned accordingly as trazodone with the combination of another CNS depressant, can result in extreme tiredness and dizziness.
Increased serum digoxin and phenytoin levels have been reported to occur in patients receiving trazodone concurrently with either of those two drugs. Little is known about the interaction between trazodone and general anesthetics; therefore, prior to elective surgery, trazodone should be discontinued for as long as clinically feasible.
Because it is not known whether an interaction will occur between trazodone and monoamine oxidase inhibitors (MAOI's), administration of trazodone should be initiated very cautiously with gradual increase in dosage as required, if an MAOI is given concomitantly or has been discontinued shortly before medication with trazodone is instituted.
Because of the absence of experience, concurrent administration of electroconvulsive therapy should be avoided.
Dosage
Trazodone adult and geriatric recommended starting dose is 150 mg once daily, it may be increased by 50 mg every three to four days. The dose may be increased slowly to a maximum of 400 mg daily for outpatients and to 600 mg daily in hospitalized patients. Pediatric initial dose should be 25 – 50 mg and may be increased up to 150 mg if necessary. Adult dosage for sedation and sleep aid is 25 – 50 mg. It is recommended that Trazodone be given in a divided dose. If necessary just a single dose, in the evening, may be given.
Overdose
Symptoms
Overdose of trazodone may cause an increase in incidence or severity of any of the reported adverse reactions, e.g. excessive sedation. Death by deliberate or accidental overdosage has been reported.[50][51] However, trazodone is often used instead of tricyclic antidepressants because it is very difficult to overdose on. Depressed patients are therefore unlikely to successfully commit suicide with trazodone.[52]
Treatment
There is no specific antidote for trazodone. Management of overdosage should, therefore, be symptomatic and supportive. Any person suspected of having taken an overdosage should be evaluated at a hospital as soon as possible. Activated charcoal, gastric lavage, and forced diuresis may be useful in facilitating elimination of the drug.
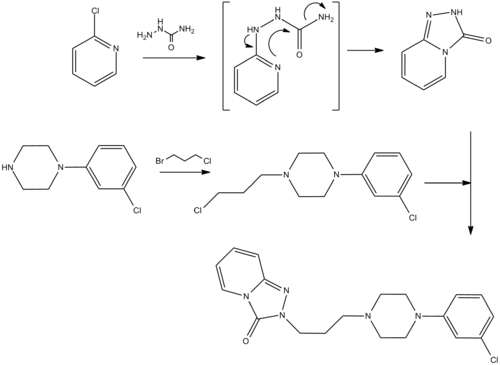
Synthesis
- Palazzo, G.; Silvestrini, B.; 1968, U.S. Patent 3,381,009.
- B. Silvestrini, G. Palazzo, DE 164594
See also
- Etoperidone
- Lubazodone
- Nefazodone
- Vilazodone
References
- ^ Haria M, Fitton A, McTavish D (April 1994). "Trazodone. A review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders". Drugs Aging 4 (4): 331–55. doi:10.2165/00002512-199404040-00006. PMID 8019056.
- ^ Silvestrini B (1989). "Trazodone: from the mental pain to the "dys-stress" hypothesis of depression". Clin Neuropharmacol 12 (Suppl 1): S4–10. PMID 2568177.
- ^ Nierenberg AA, Adler LA, Peselow E, Zornberg G, Rosenthal M (July 1994). "Trazodone for antidepressant-associated insomnia". Am J Psychiatry 151 (7): 1069–72. PMID 8010365. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=8010365.
- ^ Kaynak H, Kaynak D, Gözükirmizi E, Guilleminault C (January 2004). "The effects of trazodone on sleep in patients treated with stimulant antidepressants". Sleep Med. 5 (1): 15–20. doi:10.1016/j.sleep.2003.06.006. PMID 14725822. http://linkinghub.elsevier.com/retrieve/pii/S1389945703002065.
- ^ Scharf MB, Sachais BA (September 1990). "Sleep laboratory evaluation of the effects and efficacy of trazodone in depressed insomniac patients". J Clin Psychiatry 51 (Suppl): 13–7. PMID 2211559.
- ^ Warner,, M. D.,; Dorn,, M. R.,; Peabody, C. A., (2001). "Survey on the Usefulness of Trazodone in Patients with PTSD with Insomnia or Nightmares". Pharmacopsychiatry 34 (4): 128–131. doi:10.1055/s-2001-15871. PMID 11518472
- ^ Morillas-Arques, P; Morillas-Arques P, Rodriguez-Lopez CM, Molina-Barea R, Rico-Villademoros F, Calandre EP. (10 September 2010). "Trazodone for the treatment of fibromyalgia: an open-label, 12-week study" (Electronic only). BMC Musculoskeletal Disorders (London : BioMed Central) 11: 204. doi:10.1186/1471-2474-11-204. ISSN 1471-2474. PMC 2945951. PMID 20831796. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2945951.
- ^ Mavissakalian M, Perel J, Bowler K, Dealy R (June 1987). "Trazodone in the treatment of panic disorder and agoraphobia with panic attacks". Am J Psychiatry 144 (6): 785–7. PMID 3296792. http://ajp.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=3296792.
- ^ Rickels K, Downing R, Schweizer E, Hassman H (November 1993). "Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam". Arch. Gen. Psychiatry 50 (11): 884–95. PMID 8215814.
- ^ Pope HG, Keck PE, McElroy SL, Hudson JI (August 1989). "A placebo-controlled study of trazodone in bulimia nervosa". J Clin Psychopharmacol 9 (4): 254–9. doi:10.1097/00004714-198908000-00004. PMID 2671058.
- ^ Prasad A (February 1985). "Efficacy of trazodone as an anti obsessional agent". Pharmacol. Biochem. Behav. 22 (2): 347–8. doi:10.1016/0091-3057(85)90403-4. PMID 3983224. http://linkinghub.elsevier.com/retrieve/pii/0091-3057(85)90403-4.
- ^ Pigott TA, L'Heureux F, Rubenstein CS, Bernstein SE, Hill JL, Murphy DL (June 1992). "A double-blind, placebo controlled study of trazodone in patients with obsessive-compulsive disorder". J Clin Psychopharmacol 12 (3): 156–62. doi:10.1097/00004714-199202000-00003. PMID 1629380.
- ^ Roccatagliata G; Albano C, Maffini M, Farelli S (1980). "Alcohol withdrawal syndrome: treatment with trazodone". Int Pharmacopsychiatry. 15 (2): 105–10. PMID 6108298.
- ^ Le Bon O; Murphy JR, Staner L, Hoffmann G, Kormoss N, Kentos M, Dupont P, Lion K, Pelc I, Verbanck P (August 2003). "Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations". J Clin Psychopharmacol 23 (4): 377–83. doi:10.1097/01.jcp.0000085411.08426.d3. PMID 12920414.
- ^ Borras L; de Timary P, Constant EL, Huguelet P, Eytan A (November 2006). "Successful treatment of alcohol withdrawal with trazodone". Pharmacopsychiatry 39 (6): 232. doi:10.1055/s-2006-951385. PMID 17124647.
- ^ Hayashi T, Yokota N, Takahashi T (July 1997). "Benefits of trazodone and mianserin for patients with late-life chronic schizophrenia and tardive dyskinesia: an add-on, double-blind, placebo-controlled study". Int Clin Psychopharmacol 12 (4): 199–205. doi:10.1097/00004850-199707000-00003. PMID 9347380.
- ^ Decina P, Mukherjee S, Bocola V, Saraceni F, Hadjichristos C, Scapicchio P (December 1994). "Adjunctive trazodone in the treatment of negative symptoms of schizophrenia". Hosp Community Psychiatry 45 (12): 1220–3. PMID 7868106. http://ps.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=7868106.
- ^ Richelson E, Nelson A (July 1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". The Journal of Pharmacology and Experimental Therapeutics 230 (1): 94–102. PMID 6086881. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=6086881.
- ^ Wander TJ, Nelson A, Okazaki H, Richelson E (December 1986). "Antagonism by antidepressants of serotonin S1 and S2 receptors of normal human brain in vitro". European Journal of Pharmacology 132 (2–3): 115–21. doi:10.1016/0014-2999(86)90596-0. PMID 3816971.
- ^ Cusack B, Nelson A, Richelson E (May 1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology 114 (4): 559–65. doi:10.1007/BF02244985. PMID 7855217.
- ^ Pälvimäki EP, Roth BL, Majasuo H, et al. (August 1996). "Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor". Psychopharmacology 126 (3): 234–40. doi:10.1007/BF02246453. PMID 8876023. http://link.springer.de/link/service/journals/00213/bibs/6126003/61260234.htm.
- ^ a b Raffa RB, Shank RP, Vaught JL (1992). "Etoperidone, trazodone and MCPP: in vitro and in vivo identification of serotonin 5-HT1A (antagonistic) activity". Psychopharmacology 108 (3): 320–6. doi:10.1007/BF02245118. PMID 1387963.
- ^ a b Odagaki Y, Toyoshima R, Yamauchi T (May 2005). "Trazodone and its active metabolite m-chlorophenylpiperazine as partial agonists at 5-HT1A receptors assessed by [35SGTPgammaS binding"]. Journal of Psychopharmacology (Oxford, England) 19 (3): 235–41. doi:10.1177/0269881105051526. PMID 15888508. http://jop.sagepub.com/cgi/pmidlookup?view=long&pmid=15888508.
- ^ Tatsumi M, Groshan K, Blakely RD, Richelson E (December 1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". European Journal of Pharmacology 340 (2–3): 249–58. doi:10.1016/S0014-2999(97)01393-9. PMID 9537821. http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(97)01393-9.
- ^ a b c Marek GJ, McDougle CJ, Price LH, Seiden LS (1992). "A comparison of trazodone and fluoxetine: implications for a serotonergic mechanism of antidepressant action". Psychopharmacology 109 (1–2): 2–11. doi:10.1007/BF02245475. PMID 1365657.
- ^ Vanina Y, Podolskaya A, Sedky K, et al. (July 2002). "Body weight changes associated with psychopharmacology". Psychiatric Services (Washington, D.C.) 53 (7): 842–7. doi:10.1176/appi.ps.53.7.842. PMID 12096167. http://ps.psychiatryonline.org/cgi/pmidlookup?view=long&pmid=12096167.
- ^ Watanabe N, Omori IM, Nakagawa A, et al. (January 2010). "Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression: systematic review and meta-analysis". CNS Drugs 24 (1): 35–53. doi:10.2165/11319480-000000000-00000. PMID 20030418. http://content.wkhealth.com/linkback/openurl?issn=1172-7047&volume=24&issue=1&spage=35.
- ^ Kinney GG, Griffith JC, Hudzik TJ (July 1998). "Antidepressant-like effects of 5-hydroxytryptamine1A receptor agonists on operant responding under a response duration differentiation schedule". Behavioural Pharmacology 9 (4): 309–18. PMID 10065919.
- ^ Asayesh K (December 1986). "Combination of trazodone and phenothiazines: a possible additive hypotensive effect". Canadian Journal of Psychiatry 31 (9): 857–8. PMID 3802006.
- ^ Melzacka M, Rurak A, Vetulani J (1980). "Preliminary study of the biotransformation of two new drugs, trazodone and etoperidone". Polish Journal of Pharmacology and Pharmacy 32 (4): 551–6. PMID 7255270.
- ^ Fong MH, Garattini S, Caccia S (October 1982). "1-m-Chlorophenylpiperazine is an active metabolite common to the psychotropic drugs trazodone, etoperidone and mepiprazole". The Journal of Pharmacy and Pharmacology 34 (10): 674–5. doi:10.1111/j.2042-7158.1982.tb04701.x. PMID 6128394.
- ^ Maes M, Westenberg H, Vandoolaeghe E, et al. (October 1997). "Effects of trazodone and fluoxetine in the treatment of major depression: therapeutic pharmacokinetic and pharmacodynamic interactions through formation of meta-chlorophenylpiperazine". Journal of Clinical Psychopharmacology 17 (5): 358–64. doi:10.1097/00004714-199710000-00004. PMID 9315986. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0271-0749&volume=17&issue=5&spage=358.
- ^ Mihara K, Yasui-Furukori N, Kondo T, et al. (August 2002). "Relationship between plasma concentrations of trazodone and its active metabolite, m-chlorophenylpiperazine, and its clinical effect in depressed patients". Therapeutic Drug Monitoring 24 (4): 563–6. doi:10.1097/00007691-200208000-00016. PMID 12142643. http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0163-4356&volume=24&issue=4&spage=563.
- ^ Li AA, Marek GJ, Hand TH, Seiden LS (February 1990). "Antidepressant-like effects of trazodone on a behavioral screen are mediated by trazodone, not the metabolite m-chlorophenylpiperazine". European Journal of Pharmacology 177 (3): 137–44. doi:10.1016/0014-2999(90)90263-6. PMID 2311675.
- ^ Vetulani J, Sansone M, Baran L, Hano J (1984). "Opposite action of m-chlorophenylpiperazine on avoidance depression induced by trazodone and pimozide in CD-1 mice". Psychopharmacology 83 (2): 166–8. doi:10.1007/BF00429728. PMID 6431467.
- ^ Kast RE (2009). "Trazodone generates m-CPP: in 2008 risks from m-CPP might outweigh benefits of trazodone". The World Journal of Biological Psychiatry : the Official Journal of the World Federation of Societies of Biological Psychiatry 10 (4 Pt 2): 682–5. doi:10.1080/15622970902836022. PMID 19384678. http://informahealthcare.com/doi/abs/10.1080/15622970902836022.
- ^ Workman EA, Tellian F, Short D (May 1992). "Trazodone induction of migraine headache through mCPP". The American Journal of Psychiatry 149 (5): 712. PMID 1575270.
- ^ a b Rotzinger S, Fang J, Baker GB (1 June 1998). "Trazodone is metabolized to m-chlorophenylpiperazine by CYP3A4 from human sources". Drug Metab. Dispos. 26 (6): 572–5. PMID 9616194. http://dmd.aspetjournals.org/cgi/content/full/26/6/572.
- ^ Jauch R, Kopitar Z, Prox A, Zimmer A (1976). "[Pharmacokinetics and metabolism of trazodone in man (author's transl)] [Pharmacokinetics and metabolism of trazodone in man (author's transl)]" (in German). Arzneimittelforschung 26 (11): 2084–9. PMID 1037253.
- ^ Logan BK, Costantino AG, Rieders EF, Sanders D (Nov 2010). "Trazodone, meta-chlorophenylpiperazine (an hallucinogenic drug and trazodone metabolite), and the hallucinogen trifluoromethylphenylpiperazine cross-react with the EMIT®II ecstasy immunoassay in urine". J Anal Toxicol 34 (9): 587–9. PMID 21073812.
- ^ Webmd.com
- ^ MentalHealth.com
- ^ Warner CH, Bobo W, Warner C, Reid S, Rachal J (August 2006). "Antidepressant discontinuation syndrome". Am Fam Physician 74 (3): 449–56. PMID 16913164. http://www.aafp.org/afp/20060801/449.html.
- ^ a b c National Institutes of Health. "Trazodone Side Effects - MedlinePlus". U.S. National Library of Medicine. National Institutes of Health. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a681038.html. Retrieved 23 May 2011.
- ^ Abber, et. al. RE (1987). "Priapism induced by chlorpromazine and trazodone: mechanism of action.". J. Urol. 137 (5): 1039–1042. PMID 3573170.
- ^ Battaglia; Venturoli RE (2009). "Persistent genital arousal disorder and trazodone. Morphometric and vascular modifications of the clitoris. A case report.". J. Sex Med. 6 (10): 2896–2900. PMID 19674253.
- ^ Kalgutkar AS, Henne KR, Lame ME (June 2005). "Metabolic activation of the nontricyclic antidepressant trazodone to electrophilic quinone-imine and epoxide intermediates in human liver microsomes and recombinant P4503A4". Chem Biol Interact. 155 (1–2): 10–20. doi:10.1016/j.cbi.2005.03.036. PMID 15978881. http://linkinghub.elsevier.com/retrieve/pii/S0009-2797(05)00098-0.
- ^ Otani K, Yasui N, Kaneko S (June 1995). "Trazodone treatment increases plasma prolactin concentrations in depressed patients". Int Clin Psychopharmacol 10 (2): 115–7. doi:10.1097/00004850-199506000-00009. PMID 7673654.
- ^ Boyeson MG, Harmon RL. (October 1993). "Effects of trazodone and desipramine on motor recovery in brain-injured rats". American journal of physical medicine & rehabilitation / Association of Academic Physiatrists 72 (5): 286–93. PMID 8398020.
- ^ Martínez MA, Ballesteros S, Sánchez de la Torre C, Almarza E (2005). "Investigation of a fatality due to trazodone poisoning: case report and literature review". J Anal Toxicol 29 (4): 262–8. PMID 15975258. http://openurl.ingenta.com/content/nlm?genre=article&issn=0146-4760&volume=29&issue=4&spage=262&aulast=Martínez.
- ^ de Meester A, Carbutti G, Gabriel L, Jacques JM (2001). "Fatal overdose with trazodone: case report and literature review". Acta Clin Belg 56 (4): 258–61. PMID 11603256.
- ^ Rakel RE (1987). "The greater safety of trazodone over tricyclic antidepressant agents: 5-year experience in the United States". Psychopathology 20 (Suppl 1): 57–63. doi:10.1159/000284524. PMID 3321131.
External links
Antidepressants (N06A) Specific reuptake inhibitors (RIs), enhancers (REs), and releasing agents (RAs) Alaproclate • Citalopram • Escitalopram • Femoxetine • Fluoxetine# • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • Seproxetine • Sertraline# • Vilazodone • Zimelidine‡Bicifadine • Clovoxamine • Desvenlafaxine • Duloxetine • Levomilnacipran • Eclanamine • Milnacipran • Sibutramine • VenlafaxineSerotonin–norepinephrine–dopamine reuptake inhibitors (SNDRIs)Brasofensine • BTS-74,398 • Cocaine • Diclofensine • DOV-21,947 • DOV-102,677 • DOV-216,303 • EXP-561 • Fezolamine • JNJ-7925476 • NS-2359 • PRC200-SS • Pridefine • SEP-225,289 • SEP-227,162 • TesofensineAmedalin • Atomoxetine/Tomoxetine • Binedaline • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • ViloxazineDopamine reuptake inhibitors (DRIs)Amineptine • Bupropion/Amfebutamone# • Cilobamine • Manifaxine • Methylphenidate • Nomifensine • Radafaxine • TametralineNorepinephrine-dopamine releasing agents (NDRAs)Serotonin-norepinephrine-dopamine releasing agents (SNDRAs)4-Methyl-αMT • αET/Etryptamine • αMT/MetryptamineOthersIndeloxazine • Teniloxazine • Tramadol • ViqualineReceptor antagonists and/or reuptake inhibitors Serotonin antagonists and reuptake inhibitors (SARIs)Etoperidone • Nefazodone • TrazodoneSerotonin modulators and stimulators (SMSs)VortioxetineTricyclic and tetracyclic antidepressants (TCAs/TeCAs) TricyclicsAmezepine • Amineptine • Amitriptyline# • Amitriptylinoxide • Azepindole • Butriptyline • Cianopramine • Clomipramine • Cotriptyline • Cyanodothiepin • Demexiptiline • Depramine/Balipramine • Desipramine • Dibenzepin • Dimetacrine • Dosulepin/Dothiepin • Doxepin • Enprazepine • Fluotracen • Hepzidine • Homopipramol • Imipramine • Imipraminoxide • Intriptyline • Iprindole • Ketipramine • Litracen • Lofepramine • Losindole • Mariptiline • Melitracen • Metapramine • Mezepine • Naranol • Nitroxazepine • Nortriptyline • Noxiptiline • Octriptyline • Opipramol • Pipofezine • Propizepine • Protriptyline • Quinupramine • Tampramine • Tianeptine • Tienopramine • Trimipramine;7-OH-Amoxapine • Amoxapine • Aptazapine • Azipramine • Ciclazindol • Ciclopramine • Esmirtazapine • Loxapine • Maprotiline • Mazindol • Mianserin • Mirtazapine • Oxaprotiline • Setiptiline/TeciptilineMonoamine oxidase inhibitors (MAOIs) NonselectiveIrreversible: Benmoxin • Echinopsidine • Iproclozide • Iproniazid • Isocarboxazid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Safrazine • Tranylcypromine; Reversible: Caroxazone • Paraxazone;MAOA-SelectiveIrreversible: Clorgiline; Reversible: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Esuperone • Harmala Alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima;MAOB-SelectiveIrreversible: Ladostigil • Mofegiline • Pargyline • Rasagiline • Selegiline; Reversible: Lazabemide • MilacemideAzapirones and other 5-HT1A receptor agonists Alnespirone • Aripiprazole • Befiradol • Buspirone • Eptapirone • Flesinoxan • Flibanserin • Gepirone • Ipsapirone • Oxaflozane • Tandospirone • Vilazodone • ZalospironeHypnotics/Sedatives (N05C) GABAA Agonists/PAMs Barbiturates: Allobarbital • Amobarbital • Aprobarbital • Barbital • Butabarbital • Butobarbital • Cyclobarbital • Ethallobarbital • Heptabarbital • Hexobarbital • Mephobarbital • Methohexital • Pentobarbital • Phenobarbital • Proxibarbal • Reposal • Secobarbital • Talbutal • Thiamylal • Thiopental • Vinbarbital • Vinylbital; Benzodiazepines: Brotizolam • Clonazepam • Cinolazepam • Climazolam • Doxefazepam • Estazolam • Flunitrazepam • Flurazepam • Flutoprazepam • Haloxazolam • Loprazolam • Lormetazepam • Midazolam • Nimetazepam • Nitrazepam • Quazepam • Temazepam • Triazolam; Carbamates: Carisoprodol • Ethinamate • Hexapropymate • Meprobamate • Methocarbamol • Procymate • Tybamate; Neuroactive Steroids: Acebrochol • Allopregnanolone • Alphadolone • Alphaxolone • Eltanolone • Ganaxolone • Hydroxydione • Minaxolone • Org 20599 • Org 21465 • Tetrahydrodeoxycorticosterone; Nonbenzodiazepines: CL-218,872 • Eszopiclone • Indiplon • JM-1232 • Lirequinil • Necopidem • Pazinaclone • ROD-188 • Saripidem • Suproclone • Suriclone • SX-3228 • U-89843A • U-90042 • Zaleplon • Zolpidem • Zopiclone; Phenols: Fospropofol • Propofol; Piperidinediones: Glutethimide • Methyprylon • Pyrithyldione • Piperidione; Quinazolinones: Afloqualone • Cloroqualone • Diproqualone • Etaqualone • Mebroqualone • Mecloqualone • Methaqualone • Methylmethaqualone • Nitromethaqualone; Others: 2-Methyl-2-butanol • Acetophenone • Acetylglycinamide chloral hydrate • Bromide (Lithium bromide, Potassium bromide, Sodium bromide) • Centalun • Chloral hydrate • Chloralose • Chloralodol • Clomethiazole • Dichloralphenazone • Ethanol (Alcohol) • Ethchlorvynol • Etomidate • Gaboxadol • Loreclezole • Methylpentynol • Metomidate • Paraldehyde • Petrichloral • Sulfonmethane • Trichloroethanol • Triclofos • Valerenic acid (Valerian)GABAB Agonists H1 Inverse agonists Antihistamines: Captodiame • Cyproheptadine • Dimenhydrinate • Diphenhydramine • Doxylamine • Hydroxyzine • Methapyrilene • Pheniramine • Promethazine • Propiomazine; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α1-Adrenergic Antagonists Mianserin • Niaprazine • Trazodone; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)α2-Adrenergic Agonists 4-NEMD • Clonidine • Detomidine • Dexmedetomidine • Lofexidine • Medetomidine • Romifidine • Tizanidine • Xylazine5-HT2A Antagonists Eplivanserin • Niaprazine • Pruvanserin • Trazodone • Volinanserin; Others: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc.) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc.) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc.) • Atypical antipsychotics (Olanzapine, Quetiapine, Risperidone, etc.)Melatonin Agonists Orexin Antagonists Almorexant • SB-334,867 • SB-408,124 • SB-649,868 • Suvorexant • TCS-OX2-29Others Acecarbromal • Apronal • Bromisoval • Cannabidiol (Cannabis) • Carbromal • Embutramide • Evoxine • Fenadiazole • Gabapentin • Kavalactones (Kava) • Mephenoxalone • Opiates/Opioids (Hydrocodone, Morphine (Opium), etc.) • Passion flower • Scopolamine (Mandrake) • ValnoctamideAdrenergics Receptor ligands Agonists: 5-FNE • 6-FNE • Amidephrine • Anisodamine • Anisodine • Cirazoline • Dipivefrine • Dopamine • Ephedrine • Epinephrine (Adrenaline) • Etilefrine • Ethylnorepinephrine • Indanidine • Levonordefrin • Metaraminol • Methoxamine • Methyldopa • Midodrine • Naphazoline • Norepinephrine (Noradrenaline) • Octopamine • Oxymetazoline • Phenylephrine • Phenylpropanolamine • Pseudoephedrine • Synephrine • Tetrahydrozoline
Antagonists: Abanoquil • Adimolol • Ajmalicine • Alfuzosin • Amosulalol • Arotinolol • Atiprosin • Benoxathian • Buflomedil • Bunazosin • Carvedilol • CI-926 • Corynanthine • Dapiprazole • DL-017 • Domesticine • Doxazosin • Eugenodilol • Fenspiride • GYKI-12,743 • GYKI-16,084 • Indoramin • Ketanserin • L-765,314 • Labetalol • Mephendioxan • Metazosin • Monatepil • Moxisylyte (Thymoxamine) • Naftopidil • Nantenine • Neldazosin • Nicergoline • Niguldipine • Pelanserin • Phendioxan • Phenoxybenzamine • Phentolamine • Piperoxan • Prazosin • Quinazosin • Ritanserin • RS-97,078 • SGB-1,534 • Silodosin • SL-89.0591 • Spiperone • Talipexole • Tamsulosin • Terazosin • Tibalosin • Tiodazosin • Tipentosin • Tolazoline • Trimazosin • Upidosin • Urapidil • Zolertine
* Note that many TCAs, TeCAs, antipsychotics, ergolines, and some piperazines like buspirone, trazodone, nefazodone, etoperidone, and mepiprazole all antagonize α1-adrenergic receptors as well, which contributes to their side effects such as orthostatic hypotension.Agonists: (R)-3-Nitrobiphenyline • 4-NEMD • 6-FNE • Amitraz • Apraclonidine • Brimonidine • Cannabivarin • Clonidine • Detomidine • Dexmedetomidine • Dihydroergotamine • Dipivefrine • Dopamine • Ephedrine • Ergotamine • Epinephrine (Adrenaline) • Esproquin • Etilefrine • Ethylnorepinephrine • Guanabenz • Guanfacine • Guanoxabenz • Levonordefrin • Lofexidine • Medetomidine • Methyldopa • Mivazerol • Naphazoline • Norepinephrine (Noradrenaline) • Phenylpropanolamine • Piperoxan • Pseudoephedrine • Rilmenidine • Romifidine • Talipexole • Tetrahydrozoline • Tizanidine • Tolonidine • Urapidil • Xylazine • Xylometazoline
Antagonists: 1-PP • Adimolol • Aptazapine • Atipamezole • BRL-44408 • Buflomedil • Cirazoline • Efaroxan • Esmirtazapine • Fenmetozole • Fluparoxan • GYKI-12,743 • GYKI-16,084 • Idazoxan • Mianserin • Mirtazapine • MK-912 • NAN-190 • Olanzapine • Phentolamine • Phenoxybenzamine • Piperoxan • Piribedil • Rauwolscine • Rotigotine • SB-269,970 • Setiptiline • Spiroxatrine • Sunepitron • Tolazoline • Yohimbine
* Note that many atypical antipsychotics and azapirones like buspirone and gepirone (via metabolite 1-PP) antagonize α2-adrenergic receptors as well.βAgonists: 2-FNE • 5-FNE • Amibegron • Arbutamine • Arformoterol • Arotinolol • BAAM • Bambuterol • Befunolol • Bitolterol • Broxaterol • Buphenine • Carbuterol • Cimaterol • Clenbuterol • Denopamine • Deterenol • Dipivefrine • Dobutamine • Dopamine • Dopexamine • Ephedrine • Epinephrine (Adrenaline) • Etafedrine • Etilefrine • Ethylnorepinephrine • Fenoterol • Formoterol • Hexoprenaline • Higenamine • Indacaterol • Isoetarine • Isoprenaline (Isoproterenol) • Isoxsuprine • Labetalol • Levonordefrin • Levosalbutamol • Mabuterol • Methoxyphenamine • Methyldopa • Norepinephrine (Noradrenaline) • Orciprenaline • Oxyfedrine • Phenylpropanolamine • Pirbuterol • Prenalterol • Ractopamine • Procaterol • Pseudoephedrine • Reproterol • Rimiterol • Ritodrine • Salbutamol (Albuterol) • Salmeterol • Solabegron • Terbutaline • Tretoquinol • Tulobuterol • Xamoterol • Zilpaterol • Zinterol
Antagonists: Acebutolol • Adaprolol • Adimolol • Afurolol • Alprenolol • Alprenoxime • Amosulalol • Ancarolol • Arnolol • Arotinolol • Atenolol • Befunolol • Betaxolol • Bevantolol • Bisoprolol • Bopindolol • Bormetolol • Bornaprolol • Brefonalol • Bucindolol • Bucumolol • Bufetolol • Buftiralol • Bufuralol • Bunitrolol • Bunolol • Bupranolol • Burocrolol • Butaxamine • Butidrine • Butofilolol • Capsinolol • Carazolol • Carpindolol • Carteolol • Carvedilol • Celiprolol • Cetamolol • Cicloprolol • Cinamolol • Cloranolol • Cyanopindolol • Dalbraminol • Dexpropranolol • Diacetolol • Dichloroisoprenaline • Dihydroalprenolol • Dilevalol • Diprafenone • Draquinolol • Dropranolol • Ecastolol • Epanolol • Ericolol • Ersentilide • Esatenolol • Esmolol • Esprolol • Eugenodilol • Exaprolol • Falintolol • Flestolol • Flusoxolol • Hydroxycarteolol • Hydroxytertatolol • ICI-118,551 • Idropranolol • Indenolol • Indopanolol • Iodocyanopindolol • Iprocrolol • Isoxaprolol • Isamoltane • Labetalol • Landiolol • Levobetaxolol • Levobunolol • Levocicloprolol • Levomoprolol • Medroxalol • Mepindolol • Metalol • Metipranolol • Metoprolol • Moprolol • Nadolol • Nadoxolol • Nafetolol • Nebivolol • Neraminol • Nifenalol • Nipradilol • Oberadilol • Oxprenolol • Pacrinolol • Pafenolol • Pamatolol • Pargolol • Parodilol • Penbutolol • Penirolol • PhQA-33 • Pindolol • Pirepolol • Practolol • Primidolol • Procinolol • Pronethalol • Propafenone • Propranolol • Ridazolol • Ronactolol • Soquinolol • Sotalol • Spirendolol • SR 59230A • Sulfinalol • TA-2005 • Talinolol • Tazolol • Teoprolol • Tertatolol • Terthianolol • Tienoxolol • Tilisolol • Timolol • Tiprenolol • Tolamolol • Toliprolol • Tribendilol • Trigevolol • Xibenolol • XipranololReuptake inhibitors Selective norepinephrine reuptake inhibitors: Amedalin • Atomoxetine (Tomoxetine) • Ciclazindol • Daledalin • Esreboxetine • Lortalamine • Mazindol • Nisoxetine • Reboxetine • Talopram • Talsupram • Tandamine • Viloxazine; Norepinephrine-dopamine reuptake inhibitors: Amineptine • Bupropion (Amfebutamone) • Fencamine • Fencamfamine • Lefetamine • Levophacetoperane • LR-5182 • Manifaxine • Methylphenidate • Nomifensine • O-2172 • Radafaxine; Serotonin-norepinephrine reuptake inhibitors: Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors: Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • JNJ-7925476 • JZ-IV-10 • Methylnaphthidate • Naphyrone • NS-2359 • PRC200-SS • SEP-225,289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants: Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • melitracen • Nortriptyline • Protriptyline • Trimipramine; Tetracyclic antidepressants: Amoxapine • Maprotiline • Mianserin • Oxaprotiline • Setiptiline; Others: Cocaine • CP-39,332 • EXP-561 • Fezolamine • Ginkgo biloba • Indeloxazine • Nefazodone • Nefopam • Pridefrine • Tapentadol • Tedatioxetine • Teniloxazine • Tramadol • ZiprasidoneReleasing agents Morpholines: Fenbutrazate • Morazone • Phendimetrazine • Phenmetrazine; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Cyclazodone • Fenozolone • Fluminorex • Pemoline • Thozalinone; Phenethylamines (also amphetamines, cathinones, phentermines, etc): 2-OH-PEA • 4-CAB • 4-FA • 4-FMA • 4-MA • 4-MMA • Alfetamine • Amfecloral • Amfepentorex • Amfepramone • Amphetamine (Dextroamphetamine, Levoamphetamine) • Amphetaminil • β-Me-PEA • BDB • Benzphetamine • BOH • Buphedrone • Butylone • Cathine • Cathinone • Clobenzorex • Clortermine • D-Deprenyl • Dimethylamphetamine • Dimethylcathinone (Dimethylpropion, metamfepramone) • DMA • DMMA • EBDB • Ephedrine • Ethcathinone • Ethylamphetamine • Ethylone • Famprofazone • Fenethylline • Fenproporex • Flephedrone • Fludorex • Furfenorex • Hordenine • IAP • IMP • L-Deprenyl (Selegiline) • Lisdexamfetamine • Lophophine • MBDB • MDA (Tenamfetamine) • MDEA • MDMA • MDMPEA • MDOH • MDPEA • Mefenorex • Mephedrone • Mephentermine • Methamphetamine (Dextromethamphetamine, Levomethamphetamine) • Methcathinone • Methedrone • Methylone • NAP • Ortetamine • Paredrine • pBA • pCA • Pentorex (Phenpentermine) • Phenethylamine • Pholedrine • Phenpromethamine • Phentermine • Phenylpropanolamine • pIA • Prenylamine • Propylamphetamine • Pseudoephedrine • Tiflorex • Tyramine • Xylopropamine • Zylofuramine; Piperazines: 2C-B-BZP • BZP • MBZP • mCPP • MDBZP • MeOPP • pFPP; Others: 2-Amino-1,2-dihydronaphthalene • 2-Aminoindane • 2-Aminotetralin • 2-Benzylpiperidine • 4-Benzylpiperidine • 5-IAI • Clofenciclan • Cyclopentamine • Cypenamine • Cyprodenate • Feprosidnine • Gilutensin • Heptaminol • Hexacyclonate • Indanorex • Isometheptene • Methylhexanamine • Octodrine • Phthalimidopropiophenone • Propylhexedrine (Levopropylhexedrine) • TuaminoheptaneEnzyme inhibitors 3,4-DihydroxystyreneDBHCGS-19281A • SKF-64139 • SKF-7698Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • Tyrima; MAO-B selective: D-Deprenyl • Selegiline (L-Deprenyl) • Ladostigil • Lazabemide • Milacemide • Mofegiline • Pargyline • Rasagiline • Safinamide
* Note that MAO-B inhibitors also influence norepinephrine/epinephrine levels since they inhibit the breakdown of their precursor dopamine.COMTOthers Ferrous Iron (Fe2+) • S-Adenosyl-L-Methionine • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal Phosphate) • Vitamin B9 (Folic acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersActivity enhancers: BPAP • PPAP; Release blockers: Bethanidine • Bretylium • Guanadrel • Guanazodine • Guanclofine • Guanethidine • Guanoxan; Toxins: Oxidopamine (6-Hydroxydopamine)List of adrenergic drugsHistaminergics Receptor
ligandsAgonists: 2-Pyridylethylamine • Betahistine • Histamine • HTMT • UR-AK49
Antagonists: 1st generation: 4-Methyldiphenhydramine • Alimemazine • Antazoline • Azatadine • Bamipine • Benzatropine (Benztropine) • Bepotastine • Bromazine • Brompheniramine • Buclizine • Captodiame • Carbinoxamine • Chlorcyclizine • Chloropyramine • Chlorothen • Chlorphenamine • Chlorphenoxamine • Cinnarizine • Clemastine • Clobenzepam • Clocinizine • Cyclizine • Cyproheptadine • Dacemazine • Deptropine • Dexbrompheniramine • Dexchlorpheniramine • Dimenhydrinate • Dimetindene • Diphenhydramine • Diphenylpyraline • Doxylamine • Embramine • Etybenzatropine (Ethylbenztropine) • Etymemazine • Histapyrrodine • Homochlorcyclizine • Hydroxyethylpromethazine • Hydroxyzine • Isopromethazine • Isothipendyl • Meclozine • Mepyramine (Pyrilamine) • Mequitazine • Methafurylene • Methapyrilene • Methdilazine • Moxastine • Niaprazine • Orphenadrine • Oxatomide • Oxomemazine • Phenindamine • Pheniramine • Phenyltoloxamine • Pimethixene • Piperoxan • Promethazine • Propiomazine • Pyrrobutamine • Talastine • Thenalidine • Thenyldiamine • Thiazinamium • Thonzylamine • Tolpropamine • Tripelennamine • Triprolidine; 2nd generation: Acrivastine • Astemizole • Azelastine • Cetirizine • Clemizole • Clobenztropine • Ebastine • Emedastine • Epinastine • Ketotifen • Latrepirdine • Levocabastine • Loratadine • Mebhydrolin • Mizolastine • Olopatadine • Rupatadine • Setastine • Terfenadine; "3rd generation": Desloratadine • Fexofenadine • Levocetirizine; Miscellaneous: Tricyclic antidepressants (Amitriptyline, Doxepin, Trimipramine, etc) • Tetracyclic antidepressants (Mianserin, Mirtazapine, etc) • Serotonin antagonist and reuptake inhibitors (Trazodone, Nefazodone) • Typical antipsychotics (Chlorpromazine, Thioridazine, etc) • Atypical antipsychotics (Clozapine, Olanzapine, Quetiapine, etc)Agonists: Amthamine • Betazole • Dimaprit • Histamine • HTMT • Impromidine • UR-AK49
Antagonists: Burimamide • Cimetidine • Ebrotidine • Famotidine • Lafutidine • Lavoltidine/Loxtidine • Lupitidine • Metiamide • Niperotidine • Nizatidine • Oxmetidine • Ranitidine • RoxatidineAgonists: α-Methylhistamine • Cipralisant • Histamine • Imetit • Immepip • Immethridine • Methimepip • Proxyfan
Antagonists: A-349,821 • A-423,579 • ABT-239 • Betahistine • Burimamide • Ciproxifan • Clobenpropit • Conessine • GSK-189,254 • Impentamine • Iodophenpropit • JNJ-5,207,852 • MK-0249 • NNC-38-1,049 • PF-03654746 • Pitolisant • SCH-79,687 • Thioperamide • VUF-5,681Agonists: 4-Methylhistamine • Histamine • VUF-8,430
Antagonists: JNJ-7,777,120 • Thioperamide • VUF-6,002Reuptake
inhibitorsVMAT inhibitorsEnzyme
inhibitorsHDC inhibitorsCatechin • Meciadanol • Naringenin • TritoqualineHNMT inhibitorsDAO inhibitorsAminoguanidineOthers L-HistidineSerotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969 • Vortioxetine
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507 • VortioxetineReuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors AGN-2979 • FenclonineNonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersCategories:- Serotonin-norepinephrine reuptake inhibitors
- Serotonin antagonists
- Piperazines
- Organochlorides
- Triazolopyridines
- Ureas
- Lactams
Wikimedia Foundation. 2010.