- Ondansetron
-
Ondansetron 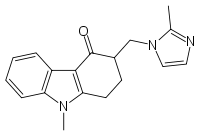
Systematic (IUPAC) name (RS)-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-2,3-dihydro-1H-carbazol-4(9H)-one Clinical data Trade names Zofran AHFS/Drugs.com monograph MedlinePlus a601209 Pregnancy cat. B1(AU) B(US) Legal status Prescription Only (S4) (AU) POM (UK) ℞-only (US) Routes Oral, rectal, IV, IM Pharmacokinetic data Bioavailability ~60% Protein binding 70%-76% Metabolism Hepatic (CYP3A4, CYP1A2, CYP2D6) Half-life 5.7 hours Excretion Renal Identifiers CAS number 99614-02-5 
ATC code A04AA01 PubChem CID 4595 DrugBank APRD00481 ChemSpider 4434 
UNII 4AF302ESOS 
KEGG D00456 
ChEMBL CHEMBL46 
Chemical data Formula C18H19N3O Mol. mass 293.4 g/mol SMILES eMolecules & PubChem  (what is this?) (verify)
(what is this?) (verify)Ondansetron (INN) (
 /ɒnˈdænsɛtrɒn/; developed and first marketed by GlaxoSmithKline as Zofran) is a serotonin 5-HT3 receptor antagonist used mainly as an antiemetic (to treat nausea and vomiting), often following chemotherapy. Its effects are thought to be on both peripheral and central nerves. Ondansetron reduces the activity of the vagus nerve, which deactivates the vomiting center in the medulla oblongata, and also blocks serotonin receptors in the chemoreceptor trigger zone. It has little effect on vomiting caused by motion sickness, and does not have any effect on dopamine receptors or muscarinic receptors.
/ɒnˈdænsɛtrɒn/; developed and first marketed by GlaxoSmithKline as Zofran) is a serotonin 5-HT3 receptor antagonist used mainly as an antiemetic (to treat nausea and vomiting), often following chemotherapy. Its effects are thought to be on both peripheral and central nerves. Ondansetron reduces the activity of the vagus nerve, which deactivates the vomiting center in the medulla oblongata, and also blocks serotonin receptors in the chemoreceptor trigger zone. It has little effect on vomiting caused by motion sickness, and does not have any effect on dopamine receptors or muscarinic receptors.Contents
Clinical uses
The 5-HT3 receptor antagonists are the primary drugs used to treat and prevent chemotherapy-induced nausea and vomiting (CINV). A common use case is to give them intravenously about 30 minutes before commencement of a chemotherapy treatment. Ondansetron is also effective in controlling post-operative nausea and vomiting (PONV) and post-radiation nausea and vomiting, and is a possible therapy for nausea and vomiting due to acute or chronic medical illness or acute gastroenteritis.
Although it is highly effective, the high cost of the brand-name version had limited its use to controlling PONV and CINV. It is also used off-label to treat hyperemesis gravidarum in pregnant women, but there is no conclusive data available on its safety in pregnancy, especially during the first trimester. It is also used to treat cyclic vomiting syndrome; although there have been no formal trials to confirm efficacy, case reports suggests it can be helpful in some cases. The drug is administered 1–3 times daily, depending on the severity of nausea and/or vomiting. The normal oral dose for adults and children over the age of 12 is 8 mg initially, followed by a second dose of 8 mg eight hours later. The drug is then administered once every 12 hours, usually for not more than 2–3 days. Following oral administration, it takes about 1.5 to 2 hours to reach maximum plasma concentrations. This drug is removed from the body by the liver and kidneys.
The clinical effect of ondansetron (and other drugs from the same group) can be potentiated by combining it with dexamethasone.
Investigational and off-label
Neuropsychiatric disorders
A 2006 double-blind, randomized controlled trial indicated that ondansetron may have value in the treatment of schizophrenia, as an adjunct to haloperidol. The study found the combination to significantly improve negative schizophrenia symptoms, and people taking both drugs experienced fewer of the adverse effects commonly associated with haloperidol.[1] An earlier, smaller, open-label trial had found ondansetron to be useful in treating antipsychotic-induced tardive dyskinesia in people with schizophrenia, and the study patients also showed significant improvement in the disease's symptoms.[2][3]
Early studies have also examined ondansetron as a possible treatment for psychosis resulting from advanced Parkinson's disease.[4] Its apparent benefits despite a lack of any significant antagonistic properties at dopamine receptors or the 5-HT2A receptor raises interesting questions about the etiology of psychosis.
Hewlett and others found that the treatment of obsessive compulsive disorder with Ondansetron 1 mg three times daily was associated with a significant decrease in the Yale Brown Obsessive Compulsive scores in a small (n=8), 8-week, open-label study.[5]
Substance use
Ondansetron lowers the cravings for alcohol, especially in early-onset alcoholics. In one cognitive-behavioral therapy study, ondansetron patients with early-onset alcoholism had fewer drinks per day and reported more days without drinking at all, as compared to the other groups in the study. Also of note, individuals with the LL genotype show significant improvements in alcohol misuse when treated with ondansetron, compared with individuals with the other genotypes of the 5HTTLPR polymorphism, who showed no improvement over placebo.[6][7][8]
Researchers at the Stanford University School of Medicine have demonstrated that ondansetron might be useful and effective for treating withdrawal symptoms of opioid addictions.[9] Unlike the existing treatments methadone and buprenorphine, it is not itself an opioid.[9] Additionally, it does not require continued supervision like treatment with clonidine.[9]
The original experiment used mice who were injected with increasing doses of morphine, assayed with naloxone and then underwent haplotypic analysis to isolate a gene candidate.[10] HTR3A which codes for the 5-HT3 receptor emerged as the primary candidate, which suggested 5-HT3 antagonist ondansetron as a possible treatment.[10] The researchers were then able to show using an acute morphine administration model the efficacy in withdrawal symptom control in humans.[10]
Irritable bowel syndrome
Ondansetron blocks the 5-HT3 receptor in the enteric nervous system, and thereby reduces colonic contractions, sensory perception, and motility. A large number of drugs in this category, 5-HT3 antagonist, have been shown to have this effect, which positively impacts irritable bowel syndrome with diarrhea (IBS-D). Thus, ondansetron has been effective in treating diarrhea-predominant IBS in initial studies, and is being used off label for this exact effect.[11]
Postanesthetic shivering
Two small, placebo-controlled trials have been conducted to assess the efficacy of ondansetron for postanesthetic shivering, a common occurrence after surgery. Ondansetron was found to be as effective as pethidine (meperidine, Demerol) when given as a single IV dose before anesthesia.[12]
Adverse effects
Ondansetron is a well-tolerated drug with few side effects. Constipation, dizziness and headache are the most commonly reported side effects associated with its use. There have been no significant drug interactions reported with this drug's use. It is broken down by the hepatic cytochrome P450 system and it has little effect on the metabolism of other drugs broken down by this system.
On September 15, 2011, the FDA issued a Medwatch Safety Alert for Zofran (ondansetron) in patients with congenital Long QT syndrome, a heart arrhythmia. The FDA further required GlaxoSmithKline to conduct a thorough QT study to determine the degree to which Zofran may cause QT interval prolongation.[1]
History
Ondansetron was developed around 1984 by scientists working at Glaxo's laboratories in London. It is in both the imidazole and carbazole families of heterocyclic compounds. After several attempts the company successfully filed for U.S. patent protection for the drug in 1986. U.S. Patent 4,695,578 was granted in September 1987 while U.S. Patent 4,753,789 was granted in June 1988. U.S. Patent 5,578,628, a divisional patent of U.S. Patent 4,753,789, was granted on November 26, 1996. Ondansetron was granted FDA approval as Zofran in January 1991. Glaxo did pediatric research on Zofran's uses, and gained a patent extension as a result, extending U.S. exclusivity until December 24, 2006. The FDA subsequently approved the first generic versions in December 2006, with marketing approval granted to Teva Pharmaceuticals USA and SICOR Pharmaceuticals.
Brand names
Ondansetron is currently marketed by GlaxoSmithKline (GSK) under the trade name Zofran. Other manufacturers include Opsonin Pharma Bngladesh (Anset), Strativa Pharmaceuticals (Zuplenz), Cipla Ltd. (Emeset), Gedeon Richter Ltd. (Emetron), Korea United Pharmaceuticals (Emodan), Zentiva a.s. (Ondemet), Strides Arcolab (Setronax), Glenmark Generics Ltd. (India) (Ondansetron) and Novell Pharmaceutical Laboratories (Ondavell). On May 29, 2006, Baxter Healthcare received tentative approval[13] to market its own label of Ondansetron Injection, USP, 8 mg/50 mL and 32 mg/50 mL iso-osmotic sodium chloride solution, beginning upon expiration of GSK's patent later that year.
References
- ^ Zhang ZJ, Kang WH, Li Q, Wang XY, Yao SM, Ma AQ (2006). "Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study". Schizophrenia Research 88 (1–3): 102–10. doi:10.1016/j.schres.2006.07.010. PMID 16959472.
- ^ Zullino DF, Eap CB, Voirol P (2001). "Ondansetron for tardive dyskinesia". Am J Psychiatry 158 (4): 657–8. doi:10.1176/appi.ajp.158.4.657-a. PMID 11282718.
- ^ Sirota P, Mosheva T, Shabtay H, Giladi N, Korczyn AD (2000). "Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia". Am J Psychiatry 157 (2): 287–9. doi:10.1176/appi.ajp.157.2.287. PMID 10671405. Free full text
- ^ Zoldan J, Friedberg G, Livneh M, Melamed E (1995). "Psychosis in advanced Parkinson's disease: treatment with ondansetron, a 5-HT3 receptor antagonist". Neurology 45 (7): 1305–8. PMID 7617188.
- ^ Hewlett WA, Schmid SP, Salomon RM (2003). "Pilot trial of ondansetron in the treatment of 8 patients with obsessive compulsive disorder". J Clin Psychiatry 64 (9): 1025–30. doi:10.4088/JCP.v64n0907. PMID 14628977.
- ^ "Ondansetron can prevent alcohol craving". June 11, 2006. http://alcoholism.about.com/cs/meds/a/aa001120a.htm. Retrieved 2007-11-05.
- ^ Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB (1994). "Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence". Alcohol Clin Exp Res 18 (4): 879–85. doi:10.1111/j.1530-0277.1994.tb00054.x. PMID 7978099.
- ^ "Genes Predict Success of Ondansetron Treatment for Alcoholism". January 25, 2011. http://www.medscape.com/viewarticle/736225. Retrieved 2011-01-25.
- ^ a b c "Stanford scientists identify drug to treat opioid addiction". 17 FEB 2009. http://med.stanford.edu/news_releases/2009/february/opioid.html. Retrieved 19 FEB 2009.
- ^ a b c Chu LF, Liang DY, Li X, Sahbaie P, Dʼarcy N, Liao G, Peltz G, David Clark J (February 2009). "From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics". Pharmacogenet. Genomics 19 (3): 193–205. doi:10.1097/FPC.0b013e328322e73d. PMC 2730361. PMID 19214139. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2730361.
- ^ Future for IBS; Medicinenet Article
- ^ Generali JA, Cada DJ (August 2009). "Ondansetron: postanesthetic shivering". Hospital Pharmacy 44 (8): 670–1. http://www.factsandcomparisons.com/assets/hpdatenamed/20090801_Aug2009_off.pdf.
- ^ "Center for Drug Evaluation & Research (FDA) letter of tentative approval for Ondansetron NDA by Baxter Healthcare Corp" (PDF). http://www.fda.gov/cder/foi/appletter/2006/021915s000TAltr.pdf.
External links
Antiemetics (A04) 5-HT3 Antagonists Alosetron • Azasetron • Bemesetron • Cilansetron • Clozapine • Dazopride • Dolasetron • Granisetron • Lerisetron • Metoclopramide • Mianserin • Mirtazapine • Olanzapine • Ondansetron • Palonosetron • Ramosetron • Ricasetron • Tropisetron • ZatosetronCB1 Agonists (Cannabinoids) D2/D3 Antagonists H1 Antagonists (Antihistamines) mACh Antagonists (Anticholinergics) NK1 Antagonists Others Serotonergics 5-HT1 receptor ligands Agonists: Azapirones: Alnespirone • Binospirone • Buspirone • Enilospirone • Eptapirone • Gepirone • Ipsapirone • Perospirone • Revospirone • Tandospirone • Tiospirone • Umespirone • Zalospirone; Antidepressants: Etoperidone • Nefazodone • Trazodone • Vortioxetine; Antipsychotics: Aripiprazole • Asenapine • Clozapine • Quetiapine • Ziprasidone; Ergolines: Dihydroergotamine • Ergotamine • Lisuride • Methysergide • LSD; Tryptamines: 5-CT • 5-MeO-DMT • 5-MT • Bufotenin • DMT • Indorenate • Psilocin • Psilocybin; Others: 8-OH-DPAT • Adatanserin • Befiradol • BMY-14802 • Cannabidiol • Dimemebfe • Ebalzotan • Eltoprazine • F-11,461 • F-12,826 • F-13,714 • F-14,679 • F-15,063 • F-15,599 • Flesinoxan • Flibanserin • Lesopitron • LY-293,284 • LY-301,317 • MKC-242 • NBUMP • Osemozotan • Oxaflozane • Pardoprunox • Piclozotan • Rauwolscine • Repinotan • Roxindole • RU-24,969 • S 14,506 • S-14,671 • S-15,535 • Sarizotan • SSR-181,507 • Sunepitron • U-92,016-A • Urapidil • Vilazodone • Xaliproden • Yohimbine
Antagonists: Antipsychotics: Iloperidone • Risperidone • Sertindole; Beta blockers: Alprenolol • Cyanopindolol • Iodocyanopindolol • Oxprenolol • Pindobind • Pindolol • Propranolol • Tertatolol; Others: AV965 • BMY-7,378 • CSP-2503 • Dotarizine • Flopropione • GR-46611 • Isamoltane • Lecozotan • Mefway • Metitepine/Methiothepin • MPPF • NAN-190 • PRX-00023 • Robalzotan • S-15535 • SB-649,915 • SDZ 216-525 • Spiperone • Spiramide • Spiroxatrine • UH-301 • WAY-100,135 • WAY-100,635 • XylamidineAgonists: Lysergamides: Dihydroergotamine • Ergotamine • Methysergide; Piperazines: Eltoprazine • TFMPP; Triptans: Avitriptan • Eletriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-MT; Others: CGS-12066A • CP-93,129 • CP-94,253 • CP-135,807 • RU-24,969 • Vortioxetine
Antagonists: Lysergamides: Metergoline; Others: AR-A000002 • Elzasonan • GR-127,935 • Isamoltane • Metitepine/Methiothepin • SB-216,641 • SB-224,289 • SB-236,057 • YohimbineAgonists: Lysergamides: Dihydroergotamine • Methysergide; Triptans: Almotriptan • Avitriptan • Eletriptan • Frovatriptan • Naratriptan • Rizatriptan • Sumatriptan • Zolmitriptan; Tryptamines: 5-CT • 5-Ethyl-DMT • 5-MT • 5-(Nonyloxy)tryptamine; Others: CP-135,807 • CP-286,601 • GR-46611 • L-694,247 • L-772,405 • PNU-109,291 • PNU-142,633
Antagonists: Lysergamides: Metergoline; Others: Alniditan • BRL-15,572 • Elzasonan • GR-127,935 • Ketanserin • LY-310,762 • LY-367,642 • LY-456,219 • LY-456,220 • Metitepine/Methiothepin • Ritanserin • Yohimbine • ZiprasidoneAgonists: Lysergamides: Methysergide; Triptans: Eletriptan; Tryptamines: BRL-54443 • Tryptamine
Antagonists: Metitepine/MethiothepinAgonists: Triptans: Eletriptan • Naratriptan • Sumatriptan; Tryptamines: 5-MT; Others: BRL-54443 • Lasmiditan • LY-334,370
Antagonists: Metitepine/Methiothepin5-HT2 receptor ligands Agonists: Lysergamides: ALD-52 • Ergometrine • Lisuride • LA-SS-Az • LSD • LSD-Pip • Lysergic acid 2-butyl amide • Lysergic acid 3-pentyl amide • Methysergide; Phenethylamines: 25I-NBF • 25I-NBMD • 25I-NBOH • 25I-NBOMe • 2C-B • 2C-B-FLY • 2CB-Ind • 2C-C-NBOMe • 2C-E • 2C-I • 2C-TFM-NBOMe • 2C-T-2 • 2C-T-7 • 2C-T-21 • 2CBCB-NBOMe • 2CBFly-NBOMe • Bromo-DragonFLY • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline • TCB-2 • TFMFly; Piperazines: BZP • Quipazine • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: AL-34662 • AL-37350A • Dimemebfe • Medifoxamine • Oxaflozane • PNU-22394 • RH-34
Antagonists: Atypical antipsychotics: Amperozide • Aripiprazole • Carpipramine • Clocapramine • Clozapine • Gevotroline • Iloperidone • Melperone • Mosapramine • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Loxapine • Pipamperone; Antidepressants: Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Mianserin • Mirtazapine • Nefazodone • Teniloxazine • Trazodone; Others: 5-I-R91150 • AC-90179 • Adatanserin • Altanserin • AMDA • APD-215 • Blonanserin • Cinanserin • CSP-2503 • Cyproheptadine • Deramciclane • Dotarizine • Eplivanserin • Esmirtazapine • Fananserin • Flibanserin • Ketanserin • KML-010 • Lubazodone • Mepiprazole • Metitepine/Methiothepin • Nantenine • Pimavanserin • Pizotifen • Pruvanserin • Rauwolscine • Ritanserin • S-14,671 • Sarpogrelate • Setoperone • Spiperone • Spiramide • SR-46349B • Volinanserin • Xylamidine • YohimbineAgonists: Oxazolines: 4-Methylaminorex • Aminorex; Phenethylamines: Chlorphentermine • Cloforex • DOB • DOC • DOI • DOM • Fenfluramine • MDA • MDMA • Norfenfluramine; Tryptamines: 5-CT • 5-MT • α-Methyl-5-HT; Others: BW-723C86 • Cabergoline • mCPP • Pergolide • PNU-22394 • Ro60-0175
Antagonists: Agomelatine • Asenapine • EGIS-7625 • Ketanserin • Lisuride • LY-272,015 • Metitepine/Methiothepin • PRX-08066 • Rauwolscine • Ritanserin • RS-127,445 • Sarpogrelate • SB-200,646 • SB-204,741 • SB-206,553 • SB-215,505 • SB-221,284 • SB-228,357 • SDZ SER-082 • Tegaserod • YohimbineAgonists: Phenethylamines: 2C-B • 2C-E • 2C-I • 2C-T-2 • 2C-T-7 • 2C-T-21 • DOB • DOC • DOI • DOM • MDA • MDMA • Mescaline; Piperazines: Aripiprazole • mCPP • TFMPP; Tryptamines: 5-CT • 5-MeO-α-ET • 5-MeO-α-MT • 5-MeO-DET • 5-MeO-DiPT • 5-MeO-DMT • 5-MeO-DPT • 5-MT • α-ET • α-Methyl-5-HT • α-MT • Bufotenin • DET • DiPT • DMT • DPT • Psilocin • Psilocybin; Others: A-372,159 • AL-38022A • CP-809,101 • Dimemebfe • Lorcaserin• Medifoxamine • MK-212 • Org 12,962 • ORG-37,684 • Oxaflozane • PNU-22394 • Ro60-0175 • Ro60-0213 • Vabicaserin • WAY-629 • WAY-161,503 • YM-348
Antagonists: Atypical antipsychotics: Clozapine • Iloperidone • Melperone • Olanzapine • Paliperidone • Pimozide • Quetiapine • Risperidone • Sertindole • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine • Pipamperone; Antidepressants: Agomelatine • Amitriptyline • Amoxapine • Aptazapine • Etoperidone • Fluoxetine • Mianserin • Mirtazapine • Nefazodone • Nortriptyline • Tedatioxetine • Trazodone; Others: Adatanserin • Cinanserin • Cyproheptadine • Deramciclane • Dotarizine • Eltoprazine • Esmirtazapine • FR-260,010 • Ketanserin • Ketotifen • Latrepirdine • Metitepine/Methiothepin • Methysergide • Pizotifen • Ritanserin • RS-102,221 • S-14,671 • SB-200,646 • SB-206,553 • SB-221,284 • SB-228,357 • SB-242,084 • SB-243,213 • SDZ SER-082 • Xylamidine5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7 ligands Agonists: Piperazines: BZP • Quipazine; Tryptamines: 2-Methyl-5-HT • 5-CT; Others: Chlorophenylbiguanide • Butanol • Ethanol • Halothane • Isoflurane • RS-56812 • SR-57,227 • SR-57,227-A • Toluene • Trichloroethane • Trichloroethanol • Trichloroethylene • YM-31636
Antagonists: Antiemetics: AS-8112 • Alosetron • Azasetron • Batanopride • Bemesetron • Cilansetron • Dazopride • Dolasetron • Granisetron • Lerisetron • Ondansetron • Palonosetron • Ramosetron • Renzapride • Tropisetron • Zacopride • Zatosetron; Atypical antipsychotics: Clozapine • Olanzapine • Quetiapine; Tetracyclic antidepressants: Amoxapine • Mianserin • Mirtazapine; Others: CSP-2503 • ICS-205,930 • MDL-72,222 • Memantine • Nitrous Oxide • Ricasetron • Sevoflurane • Tedatioxetine • Thujone • Vortioxetine • XenonAgonists: Gastroprokinetic Agents: Cinitapride • Cisapride • Dazopride • Metoclopramide • Mosapride • Prucalopride • Renzapride • Tegaserod • Velusetrag • Zacopride; Others: 5-MT • BIMU8 • CJ-033,466 • PRX-03140 • RS-67333 • RS-67506 • SL65.0155 • Antagonists: GR-113,808 • GR-125,487 • L-Lysine • Piboserod • RS-39604 • RS-67532 • SB-203,186 • SB-204,070Agonists: Lysergamides: Ergotamine • LSD; Tryptamines: 5-CT; Others: Valerenic Acid
Antagonists: Asenapine • Latrepirdine • Metitepine/Methiothepin • Ritanserin • SB-699,551
* Note that the 5-HT5B receptor is not functional in humans.Agonists: Lysergamides: Dihydroergotamine • Ergotamine • Lisuride • LSD • Mesulergine • Metergoline • Methysergide; Tryptamines: 2-Methyl-5-HT • 5-BT • 5-CT • 5-MT • Bufotenin • E-6801 • E-6837 • EMD-386,088 • EMDT • LY-586,713 • Tryptamine; Others: WAY-181,187 • WAY-208,466
Antagonists: Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Doxepin • Mianserin • Nortriptyline; Atypical antipsychotics: Aripiprazole • Asenapine • Clozapine • Fluperlapine • Iloperidone • Olanzapine • Tiospirone; Typical antipsychotics: Chlorpromazine • Loxapine; Others: BGC20-760 • BVT-5182 • BVT-74316 • Cerlapirdine • EGIS-12,233 • GW-742,457 • Ketanserin • Latrepirdine • Lu AE58054 • Metitepine/Methiothepin • MS-245 • PRX-07034 • Ritanserin • Ro04-6790 • Ro 63-0563 • SB-258,585 • SB-271,046 • SB-357,134 • SB-399,885 • SB-742,457Agonists: Lysergamides: LSD; Tryptamines: 5-CT • 5-MT • Bufotenin; Others: 8-OH-DPAT • AS-19 • Bifeprunox • E-55888 • LP-12 • LP-44 • RU-24,969 • Sarizotan
Antagonists: Lysergamides: 2-Bromo-LSD • Bromocriptine • Dihydroergotamine • Ergotamine • Mesulergine • Metergoline • Methysergide; Antidepressants: Amitriptyline • Amoxapine • Clomipramine • Imipramine • Maprotiline • Mianserin; Atypical antipsychotics: Amisulpride • Aripiprazole • Clozapine • Olanzapine • Risperidone • Sertindole • Tiospirone • Ziprasidone • Zotepine; Typical antipsychotics: Chlorpromazine • Loxapine; Others: Butaclamol • EGIS-12,233 • Ketanserin • LY-215,840 • Metitepine/Methiothepin • Pimozide • Ritanserin • SB-258,719 • SB-258,741 • SB-269,970 • SB-656,104 • SB-656,104-A • SB-691,673 • SLV-313 • SLV-314 • Spiperone • SSR-181,507 • VortioxetineReuptake inhibitors Selective serotonin reuptake inhibitors (SSRIs): Alaproclate • Citalopram • Dapoxetine • Desmethylcitalopram • Desmethylsertraline • Escitalopram • Femoxetine • Fluoxetine • Fluvoxamine • Indalpine • Ifoxetine • Litoxetine • Lubazodone • Panuramine • Paroxetine • Pirandamine • RTI-353 • Seproxetine • Sertraline • Tedatioxetine • Vilazodone • Vortioxetine • Zimelidine; Serotonin-norepinephrine reuptake inhibitors (SNRIs): Bicifadine • Desvenlafaxine • Duloxetine • Eclanamine • Levomilnacipran • Milnacipran • Sibutramine • Venlafaxine; Serotonin-norepinephrine-dopamine reuptake inhibitors (SNDRIs): Brasofensine • Diclofensine • DOV-102,677 • DOV-21,947 • DOV-216,303 • NS-2359 • SEP-225289 • SEP-227,162 • Tesofensine; Tricyclic antidepressants (TCAs): Amitriptyline • Butriptyline • Cianopramine • Clomipramine • Desipramine • Dosulepin • Doxepin • Imipramine • Lofepramine • Nortriptyline • Pipofezine • Protriptyline • Trimipramine; Tetracyclic antidepressants (TeCAs): Amoxapine; Piperazines: Nefazodone • Trazodone; Antihistamines: Brompheniramine • Chlorphenamine • Diphenhydramine • Mepyramine/Pyrilamine • Pheniramine • Tripelennamine; Opioids: Pethidine • Methadone • Propoxyphene; Others: Cocaine • CP-39,332 • Cyclobenzaprine • Dextromethorphan • Dextrorphan • EXP-561 • Fezolamine • Mesembrine • Nefopam • PIM-35 • Pridefine • Roxindole • SB-649,915 • ZiprasidoneReleasing agents Aminoindanes: 5-IAI • AMMI • ETAI • MDAI • MDMAI • MMAI • TAI; Aminotetralins: 6-CAT • 8-OH-DPAT • MDAT • MDMAT; Oxazolines: 4-Methylaminorex • Aminorex • Clominorex • Fluminorex; Phenethylamines (also Amphetamines, Cathinones, Phentermines, etc): 2-Methyl-MDA • 4-CAB • 4-FA • 4-FMA • 4-HA • 4-MTA • 5-APDB • 5-Methyl-MDA • 6-APDB • 6-Methyl-MDA • AEMMA • Amiflamine • BDB • BOH • Brephedrone • Butylone • Chlorphentermine • Cloforex • Amfepramone • Metamfepramone • DCA • DFMDA • DMA • DMMA • EBDB • EDMA • Ethylone • Etolorex • Fenfluramine (Dexfenfluramine) • Flephedrone • IAP • IMP • Lophophine • MBDB • MDA • MDEA • MDHMA • MDMA • MDMPEA • MDOH • MDPEA • Mephedrone • Methedrone • Methylone • MMA • MMDA • MMDMA • MMMA • NAP • Norfenfluramine • 4-TFMA • pBA • pCA • pIA • PMA • PMEA • PMMA • TAP; Piperazines: 2C-B-BZP • 2-BZP • 3-MeOPP • BZP • DCPP • MBZP • mCPP • MDBZP • MeOPP • Mepiprazole • pCPP • pFPP • pTFMPP • TFMPP; Tryptamines: 4-Methyl-αET • 4-Methyl-αMT • 5-CT • 5-MeO-αET • 5-MeO-αMT • 5-MT • αET • αMT • DMT • Tryptamine (itself); Others: Indeloxazine • Tramadol • ViqualineEnzyme inhibitors Nonselective: Benmoxin • Caroxazone • Echinopsidine • Furazolidone • Hydralazine • Indantadol • Iproclozide • Iproniazid • Isocarboxazid • Isoniazid • Linezolid • Mebanazine • Metfendrazine • Nialamide • Octamoxin • Paraxazone • Phenelzine • Pheniprazine • Phenoxypropazine • Pivalylbenzhydrazine • Procarbazine • Safrazine • Tranylcypromine; MAO-A Selective: Amiflamine • Bazinaprine • Befloxatone • Befol • Brofaromine • Cimoxatone • Clorgiline • Esuprone • Harmala alkaloids (Harmine, Harmaline, Tetrahydroharmine, Harman, Norharman, etc) • Methylene Blue • Metralindole • Minaprine • Moclobemide • Pirlindole • Sercloremine • Tetrindole • Toloxatone • TyrimaOthers Ferrous iron (Fe2+) • Magnesium (Mg2+) • Tetrahydrobiopterin • Vitamin B3 (Niacin, Nicotinamide → NADPH) • Vitamin B6 (Pyridoxine, Pyridoxamine, Pyridoxal → Pyridoxal phosphate) • Vitamin B9 (Folic Acid → Tetrahydrofolic acid) • Vitamin C (Ascorbic acid) • Zinc (Zn2+)OthersCategories:- Antiemetics
- 5-HT3 antagonists
- Imidazoles
- GlaxoSmithKline
- Ketones
Wikimedia Foundation. 2010.